Maria J Vale MD1*, Pedro Caldes MD1, Liliana Coutinho MD2
1HSM-ULS Guarda
2HST – ULS Viseu Dão-Lafões
*Corresponding Author: Maria João Vale, HSM-ULS Guarda. Orcid: 0000-0003-1386-1371.
Abstract
The surgical approach to complicated acute diverticulitis has evolved, often avoiding emergency surgery. This rare case highlights how standard protocols for suspected complicated acute diverticulitis may not always be optimal. It encourages clinicians to reflect on their approach, enhancing clinical awareness and thoughtful decision-making.
A 71-year-old patient with a history of total hysterectomy and bilateral salpingo-oophorectomy 24 years prior presented with abdominal pain and fever. Laboratory tests revealed elevated inflammatory markers, and a CT scan showed a heterogeneous intra-abdominal lesion. The initial suspicion was complicated acute diverticulitis (Hinchey I). Computed tomography-guided percutaneous drainage was performed. The persistence of the lesion and diagnostic uncertainty led to an excisional biopsy being performed. Histopathology revealed a low-grade uterine stromal sarcoma. This unusual presentation—characterized by a solitary uterine stromal sarcoma in an atypical location, with no history of endometriosis and no malignancy in the previous hysterectomy and oophorectomy (24 years ago)—highlights the importance of considering neoplastic origins in similar cases.
Although percutaneous drainage is commonly used, it may have been counterproductive in this case, risking tumor dissemination and potentially worsening the prognosis.
Keywords: Acute Diverticulitis, Percutaneous Drainage, Surgery, Extrauterine Stromal Sarcoma, Case Report.
Introduction
Endometrial stromal sarcomas are rare, comprising only about 0.2% of all uterine malignancies but accounting for 7–25% of uterine sarcomas [1]. Low-grade endometrial stromal sarcoma (LG-ESS) is the most prevalent subtype, typically located in the uterine body but occasionally appearing in the ovary, pelvis, abdominal cavity, vulva, or vagina [2]. Extrauterine presentation is rare and, in 50% of cases, linked to endometriosis.
This case is not only rare but also challenges the conventional management of suspected complicated acute diverticulitis, prompting a reassessment of standard approaches to enhance clinical insight and response.
Material and Methods
A 71-year-old caucasian female presented to the emergency department with constant left flank pain and fever, persisting for one day, with no associated symptoms like nausea, vomiting, or genitourinary/intestinal issues. Her history included a total hysterectomy and bilateral salpingo-oophorectomy 24 years prior (an anatomopathological study revealed leiomyomas). She was on transdermal hormone therapy (estradiol 25μg/24h) and had no other significant medical, surgical, or familial history. She had a recent colonoscopy report with no pathological alterations.
On examination, the patient was febrile (38.2°C) with a painful left flank mass, though without signs of peritoneal irritation. Laboratory tests indicated leukocytosis (12790/μL), neutrophilia (9770/μL), elevated C-reactive protein (21.48 mg/dL), and increased lactate dehydrogenase (305 U/L). "A contrast-enhanced abdominopelvic CT scan revealed a heterogeneous lesion with a cystic component located in the anterior region of the flank, near the transition to the left iliac fossa (neo formative lesion?). The lesion measured approximately 6.2 cm and was associated with a small amount of intraperitoneal fluid (Figure 1)
Despite initial uncertainty, a diagnosis of complicated acute sigmoid diverticulitis (Hinchey I) was reached after consultation with interventional radiology. CT-guided percutaneous drainage was performed, yielding about 5 mL of cloudy fluid.
Microbiological analysis showed leukocytes with no bacteria or fungi. The patient was admitted on meropenem and showed clinical improvement. Subsequent ultrasound confirmed the drain's position but indicated a similar-sized, multilobulated collection.
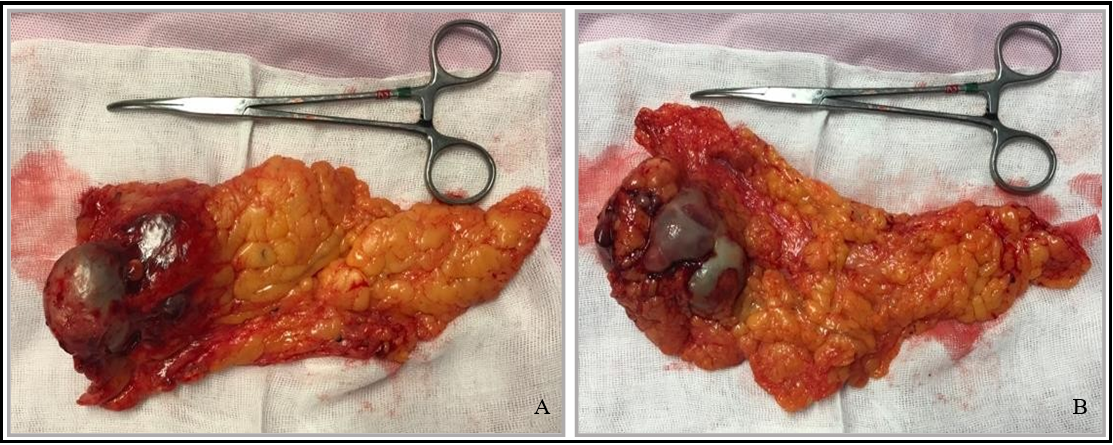
A CT scan was requested for better characterization: "The lesion is still overlapping... it has small solid components and two faint calcifications; percutaneous drainage placed inside." The patient again agreed to an exploratory laparotomy, which revealed a tumor mass in the greater omentum, adherent to the abdominal wall and small intestine, which was excised (Figure 2).
Figure 1: CT sections. A - coronal and B - Axial sections
Figure 2: Surgical specimen. A - Anterior and B - Posterior side
Results
Histopathology revealed spindle cell sarcoma consistent with endometrial stromal sarcoma, measuring 7x6x4.2 cm. The postoperative course was uneventful, and the patient was discharged on the 3rd postoperative day.
The CT scan, Pet-Scan, and staging laparoscopy did not show signs of metastization. The clinical case was presented at a multidisciplinary meeting, and it was decided to stop the transdermal hormone therapy and surveillance. The Follow-up appointments were scheduled every 3 months during the first two years and then every 6 months, with a CT scan every 6 months for the first 2 years and then every 12 months. Three years post- surgery, the patient remains asymptomatic, with no signs of recurrence.
Discussion
Low-grade endometrial stromal sarcoma (LG-ESS) generally manifests during premenopausal years (average age 52), often presenting with abnormal vaginal bleeding, uterine enlargement, or associated symptoms such as lower abdominal pain. Risk factors include obesity, diabetes, early menarche, hyperestrogenism, and tamoxifen use [5,6]. LG-ESS tumors have an indolent course with late recurrence (some 20 years later), often associated with morcellation during hysterectomy (56%), complicating diagnosis, which typically occurs post-hysterectomy for presumed benign conditions.
In this case, no indications suggested LG-ESS; prior hysterectomy and oophorectomy histology revealed no malignancy. Extrauterine LG-ESS is rare, especially in patients without endometriosis [4]. Malignant transformation of endometriotic implants is uncommon and rarely exhibits endometrial stromal sarcoma histology [3].
Staging remains the key prognostic factor. Four stages are defined according to the FIGO and TNM classifications. At diagnosis, 65% of tumors are grade I/T1-II/T2 (5-year survival > 90%) and 35% grade III/T3-IV/T4 (5-year survival 50%). [1] Here, staging was challenging; if this tumor was indeed primary (given the absence of malignancy in previous histology), it would qualify as stage Ib (tumor >5 cm limited to omentum) or stage IVa (adjacent organ invasion due to percutaneous drainage). Since laparoscopy did not reveal peritoneal carcinomatosis, the tumor was staged as Ib.
Surgical resection remains the treatment of choice for LG-ESS stages I-II. Radiotherapy is usually not recommended in low-grade sarcomas but may be used for local recurrence, which was not observed in this case. In cases of oligometastatic disease, surgical excision of lesions is preferred when feasible. Alternatively, other ablative therapies, such as stereotactic body radiation therapy, may be considered. Patients with a prolonged disease-free interval (typically at least 12 to 18 months) and a single-site recurrence are most likely to benefit from these approaches. After complete excision of metastases, the role of systemic treatment remains controversial and is best determined on a case-by-case basis [7].
A total hysterectomy and bilateral salpingo-oophorectomy are the mainstay of treatment for LG-ESS I-II. Given that the patient had already undergone the procedure, it was decided to keep her under surveillance.
The recommended follow-up includes a physical examination every 3 months for the first 2 years, then every 6 months until age 5, and annually until age 10. Thoracic, abdominal, and pelvic CT scans (or thoracic CT with abdominal and pelvic MRI) are advised every 6 months for the first 3 years, followed by annual scans for an additional 2 years. Extending annual imaging assessments to 10 years may also be considered. In selected cases, PET-CT is recommended if metastases are suspected. Additional evaluations should be guided by specific symptoms or clinical signs of metastatic disease.[7]
Conclusions
Neoplastic masses should always be considered in differential diagnoses. Surgery is the gold standard for diagnosing/treating this type of lesion, especially in the absence of unrespectability criteria. Percutaneous drainage is often counterproductive in cases like this, risking tumor spread and potentially worsening the prognosis.
This case illustrates that advancements in diagnostic and treatment modalities are invaluable but cannot replace a thorough, multidisciplinary clinical approach.
Contribution:
Maria J Vale1,2,3, Pedro Caldes2,3, Liliana Coutinho2,3
1Substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data;
2Drafting the article or revising it critically for important intellectual content;
3Final approval of the version to be published.
References
- Thiel F.C., Halmen S (2018) Low-Grade Endometrial Stromal Sarcoma - a Review. Oncology research and treatment. 41(11): 687–692.
- Conklin C.M., Longacre T.A (2014) Endometrial stromal tumors: the new WHO classification. Advances in anatomic pathology. 21(6): 383–393.
- Clair K, Wolford J, Veran-Taguibao S, Kim G, Eskander RN (2017) Primary low-grade endometrial stromal sarcoma of the omentum. Gynecologic oncology reports. 21: 119–121.
- Lu Y, Huang B, Lai C, Wang K, Yen M, et al. (2014) Endometrial stromal sarcoma occurring 20 years after total hysterectomy for myomas. Gynecology and Minimally Invasive Therapy. 3(1): 19-22.
- Zappacosta R, Fanfani F, Zappacosta B, Sablone F, Pansa L, Liberati M, et al. (2018) Uterine Sarcomas: An Updated Overview [Internet]. Neoplasm. InTech.
- Puliyath G, Nair M.K (2012) Endometrial stromal sarcoma: A review of the literature. Indian journal of medical and paediatric oncology. 33(1): 1–6.
- Pregal A., et al. (2020) Consensos nacionais de cancro ginecológico. Sociedade Portuguesa de Ginecologia.