Chun Peng Goh1*, Ting Ting Feng2, Su Lone Lim2, Ira Siyang Sun2, Shiong Wen Low2
1Division of Neurosurgery, Department of General Surgery, National University Hospital, Singapore.
2Division of Neurosurgery, Department of General Surgery, Ng Teng Fong General Hospital, Singapore.
*Corresponding Author: Chun Peng Goh, Division of Neurosurgery, Department of General Surgery, National University Hospital, Singapore.
Abstract
Isolated oculomotor nerve palsy is usually suggestive of a rapidly expanding posterior communicating artery aneurysm. We report five patients who presented with isolated oculomotor nerve palsy which was secondary to pituitary apoplexy. Oculomotor nerve function in these five patients improved after early surgical decompression. The postulated mechanisms by which pituitary apoplexy causes oculomotor nerve palsy are discussed. This article emphasizes the importance of recognizing pituitary apoplexy as a cause of isolated oculomotor cranial nerve palsy.
Keywords: Pituitary apoplexy, oculomotor nerve palsy
Introduction
The first case of pituitary tumour haemorrhage was described by Pearce Bailey in 1898 [1], but the first full description was published in 1950 by Brougham et al, using the term “pituitary apoplexy” [3]. Pituitary apoplexy is a potentially fatal medical condition characterized by a sudden onset of headache, visual symptoms (visual impairment, visual field defects, ophthalmoplegia), altered mental status, and hormonal dysfunction due to acute haemorrhage, and/or necrosis or infarction of a pituitary gland [2,4,5]. A pre-existing pituitary adenoma is usually present [2,6], but infarction may also occur in an apparently normal gland, such as in Sheehan Syndrome [7]. The visual symptoms may include both visual acuity impairment and visual field impairment from the involvement of the optic nerve or chiasm and ocular motility dysfunction from the involvement of the cranial nerves traversing the cavernous sinus [2]. An expanding mass in the cavernous sinus can compress cranial nerves III, IV, V and VI, thereby producing various degrees of cranial nerve palsy [8]. However, isolated third cranial nerve palsy as the presenting sign of pituitary apoplexy is rare and is more suggestive of a rapidly expanding posterior communicating artery aneurysm. There are several case series describing isolated third cranial nerve palsy from pituitary apoplexy [9-17]. However, the exact underlying pathophysiology remains unknown.
We recorded 5 cases of pituitary apoplexy presenting as isolated third cranial nerve palsy between 2015 and 2019 from three neurosurgical institutions in Singapore. One of these cases will be elaborated on below. We also did a review of the literature to look for possible underlying pathophysiological mechanisms leading to this rare presentation of pituitary apoplexy.
Case 1
A 61-year-old male presented with sepsis secondary to acute gangrenous cholecystitis and was admitted to intensive care unit for stabilisation prior to emergency cholecystectomy. Neurosurgery was consulted for a new-onset anisocoria.
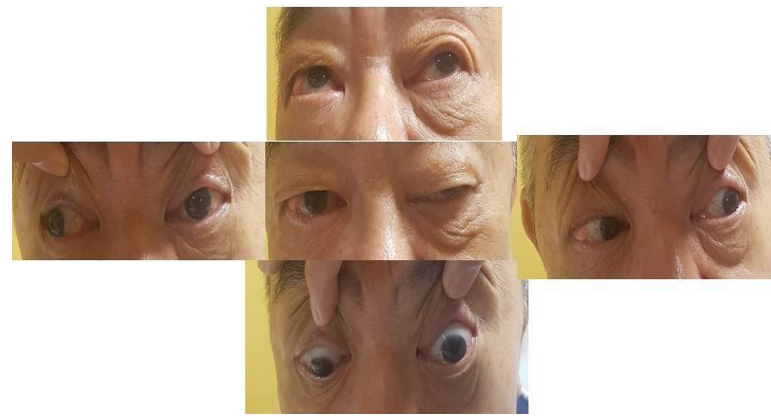
He complained of bi-frontal headache and developed an acute onset of partial ptosis of the left eye and anisocoria. In-room light, the left pupil measured 3 mm and was not reactive to direct light. He demonstrated the weakness of his left superior and inferior recti on lateral gaze, but no classic ‘down and out’ on central gaze (Figure 1). His visual field on confrontation was normal.
Figure 1: Clinical examination demonstrated left third cranial nerve palsy.
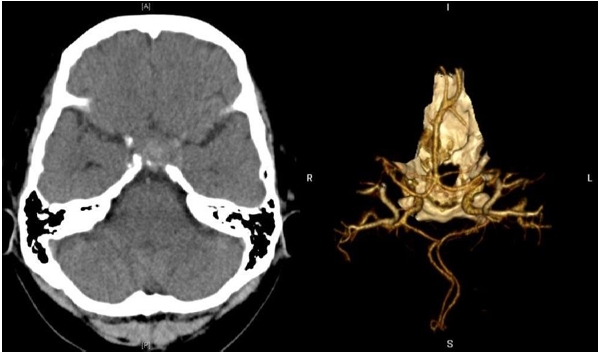
Based on the presence of the left third cranial nerve palsy and headache, our initial concern was an expanding aneurysm of the posterior communicating artery. Computed Tomography (CT) angiogram (Figure 2) did not demonstrate an aneurysm but showed a dumbbell-shaped seller mass with suprasellar extension, measuring 2.2 x 2.0 x 2.3 cm in size, causing expansion of the Sella.
Figure 2: Non-enhanced computed tomography (CT) showed an intrasellar mass without evidence of subarachnoid haemorrhage. CT angiography shows no evidence of an intracerebral aneurysm.
After an emergency laparoscopic cholecystectomy, Magnetic resonance imaging (MRI) of the head and seller region was delayed for two days as he remained hypotensive after the surgery. He developed diabetes insipidus during this period and was commenced on oral desmopressin 50mcg daily. He was started on intravenous hydrocortisone 100mg 6 hourlies for his hypocortisolism. His urinary output improved, and his blood pressure normalised.
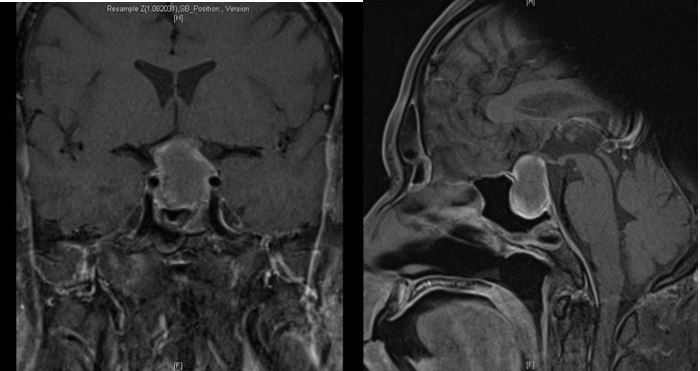
Subsequent MRI revealed subacute haemorrhage in the pituitary gland, with underlying pituitary macroadenoma with both solid and cystic components. The mass abuts the optic chiasm, with likely optic neuropathy involving the optic chiasm and both optic nerves as well as Knosp 1 left cavernous sinus extension (Figure 3).
Figure 3: Gadolinium-enhanced T1 weighted coronal and sagittal MRI of the brain showing subacute haemorrhage within an underlying 2.2 x 2 x 2.3cm-sized pituitary macroadenoma. The mass abuts the optic chiasm with Knosp 1 left cavernous sinus extension.
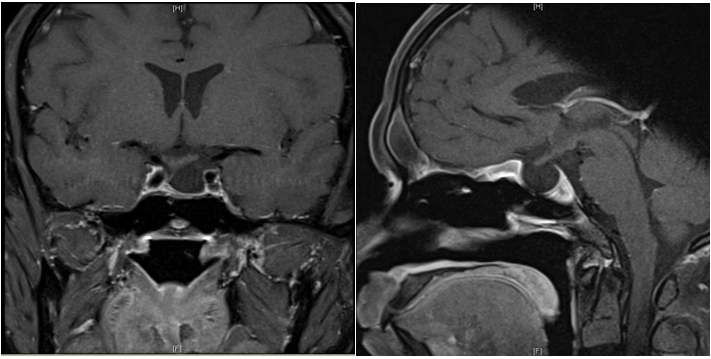
A diagnosis of pituitary apoplexy was made, and neurosurgical decompression was performed via endoscopic trans-sphenoidal approach. Histology confirmed a pituitary adenoma with areas of haemorrhage consistent with apoplexy. Post-surgery, his left third cranial nerve palsy has completely recovered. A formal visual field examination with a Humphrey Field Analyzer (HFA) showed normal results. He developed pan-hypopituitarism and diabetes insipidus persisted. He was maintained on hormonal replacements including desmopressin, hydrocortisone, thyroxine, and intramuscular testosterone. He was discharged well on the seventh postoperative day. He was reviewed in 3 months after surgery with a repeat MRI (Figure 4).
Figure 4: Gadolinium-enhanced T1 weighted coronal and sagittal MRI of the brain after endoscopic trans-sphenoidal resection of the pituitary tumour.
Table 1: Summary of five cases, with patient’s demographic, presenting symptoms, clinical and radiological findings, treatment received, and outcome/time to recovery.
|
Case |
Age |
Sex |
Presenting symptoms |
Degree of CN III palsy |
Time from diagnosis to surgery |
Radiological findings |
Knosp classifi cation |
Treatment |
Outcome of CN III palsy |
Time to recovery |
|
1 |
61 |
M |
Headache, diplopia, ptosis |
Partial |
1 week |
2.2 x 2 x 2.3cm pituitary macroadenoma abutting optic chiasm |
1 |
Endoscopic trans- sphenoidal resection |
Full recovery |
1 day |
|
2 |
69 |
M |
Headache, diplopia, vertigo |
Complete |
1 week |
1.7 x 1.6 x 1.6 cm pituitary macroadenoma displacing optic chiasm superiorly. |
2 |
Endoscopic trans-sphenoidal resection |
Full recovery |
1 week |
|
3 |
60 |
M |
Retro-orbital pain, vomiting, diplopia |
Complete |
2 weeks |
1.7 x 2.4 x 2.5 cm pituitary macroadenoma and displacing optic chiasm superiorly. |
3 |
Endoscopic trans-sphenoidal resection |
Full recovery |
1 day |
|
4 |
65 |
M |
Headache, diplopia. |
Partial progressed to complete |
4 days |
2.3 x 1.2cm pituitary macroadenoma, no mass effect on optic chiasm. |
3 |
Endoscopic trans-sphenoidal resection |
Partial recovery |
Months |
|
5 |
83 |
M |
Headache, diplopia |
Complete |
2 weeks |
2.7cm x 2.0cm x 2.3cm pituitary macroadenoma displacing optic chiasm superiorly. |
2 |
Endoscopic trans-sphenoidal resection |
Partial recovery |
Months |
Discussion
Pituitary apoplexy is an acute haemorrhagic or ischemic vascular event often involving a pituitary adenoma or the pituitary gland itself. Pituitary adenomas comprise approximately 10 % of intracranial tumors [18], and it is well recognised that they are particularly prone to haemorrhage and necrosis. The exact incidence of pituitary apoplexy is difficult to estimate as the diagnostic criteria may vary, and many cases remain undiagnosed until they present with pituitary apoplexy [6, 19]. Most series indicate that the incidence of apoplexy in pituitary adenomas is between 2 % and 7 % when defined based on clinical signs with surgical or histopathological evidence [2, 20-22]. The presentation of pituitary apoplexy is highly variable ranging from acute severe symptoms with rapid deterioration, to subacute with slowly evolving over days to weeks [22], making it a diagnostic challenge. The most common symptom is sudden onset severe headache, sometimes associated with visual disturbances such as visual acuity impairment, visual field defects, or ocular palsy resulting in diplopia and ophthalmoplegia. Signs of meningeal Table 2 irritation such as neck pain, rigidity, and photophobia may be present. In some cases, altered consciousness may be present, which can progress to coma or death if not diagnosed and treated promptly.
Isolated third cranial nerve palsy as the presenting sign of pituitary apoplexy is very rare, with limited case reports/case serious been reported in the literature [9-17]. The presenting symptoms (sudden headache, visual impairment, and ophthalmoplegia) of pituitary apoplexy together with third cranial nerve palsy can mimic several other conditions, such as subarachnoid haemorrhage due to an aneurysm (classically an enlarging posterior communicating artery aneurysm), bacterial meningitis, cavernous sinus thrombosis, and midbrain infarction [4,23], making the diagnosis difficult and often delayed, especially if the underlying pituitary adenoma was unknown. Lloyd et al [24] has summarised the clinical symptoms and signs of pituitary apoplexy based on the underlying pathological changes (Table 2).
|
Pathological Change |
Clinical sequelae |
|
Leakage of blood into subarachnoid space |
Features of subarachnoid haemorrhage |
|
Leakage of necrotic tissue into subarachnoid space |
Features of pyogenic meningitis |
|
Destruction of pituitary tissue |
Hypopituitarism |
|
Pressure on: Optic chiasm and tracts Cranial nerves 3, 4 and 6 Internal carotid and its branches Hypothalamus |
Visual field defects, impaired visual acuity Ocular palsies Hemiplegia Hyperpyrexia, mental confusion, impaired water balance |
The exact mechanism of isolated third cranial nerve palsy in pituitary apoplexy remains uncertain. We reviewed a few published reports and their proposed possible underlying mechanisms/pathophysiology.
The oculomotor nucleus is in the midbrain, consisting of the main motor nucleus, as well as the Edinger- Westphal nucleus (accessory parasympathetic nucleus). The nerve passes through the interpeduncular fossa and then between the posterior cerebral artery and the superior cerebellar artery before it reaches the cavernous sinus. The nerve then divides into superior and inferior divisions to enter the orbit. It is worth noting that the fibers innervation the pupillary muscles (pupillomotor fibers) are located superficially in the nerve trunk, being supplied by the pila vessels, while the main trunk is supplied by the vasa vasorum [25]. As a result, lesions that compress the nerve externally will compress the pupillomotor fibers and their blood supply, causing pupil dilation. In contrast, medical causes such as diabetes mellitus affecting the vasa vasorum will result in pupil-sparing third nerve palsy.
Compression or damage of the third cranial nerve causing palsy can occur anywhere in its course from the nucleus in the dorsal mesencephalon, its fascicles in the brainstem parenchyma, the nerve root in subarachnoid space, or in the cavernous sinus or posterior orbit [2, 25]. It (CN III) travels through the superior, lateral aspect of the cavernous sinus, at approximately the same horizontal level as the pituitary gland [26]. Due to its location within the cavernous sinus, the third cranial nerve is relatively more susceptible to laterally transmitted pressure generated by an expanding pituitary mass abutting the cavernous sinus [16, 17]. Kawase’s cadaveric dissection showed that one of the weak points in the cavernous sinus wall is at the meningeal pockets of the oculomotor and trochlear nerves. The dural layer is extremely thin or missing within the pockets which provides a path of least resistance to tumour expansion. Hence the oculomotor nerve is at risk of damage by the bulging of the tumour at this point [27]. On the other hand, the trochlear nerve is tightly covered with the tentorial fold at the portion posterior to the dural pocket; hence it is the least commonly affected in pituitary tumours.
Hence, a large pituitary mass may compress the third cranial nerve within the lateral wall of the cavernous sinus, or the mass may directly invade through the sinus wall. However, such mechanical compression of the third cranial nerve against the cavernous sinus wall tends to occur late during tumour growth, leading to slow-onset nerve palsy [21, 28]. In contrast, sudden onset third nerve palsy is likely the result of acute haemorrhage or infarct, leading to compromised vascular supply of the nerve due to compression of the vasa nervorum originating in the internal carotid artery [10, 28, 29]. In such cases, we would expect pupillary dilation to be present as well, as the pupillomotor nerve fibers are located superficially [25, 30]. Lau et al postulated that direct tumour invasion of the cavernous sinus is not required for ocular palsy. An enlarged Sella implies that the tumour is closer to the oculomotor nerve, causing it to be more vulnerable to the effects of transmitted pressure and early tumour invasion from within the Sella turcica [9, 15].
Kobayashi et al explained that the oculomotor nerve palsy may be caused first by unilateral erosion of the posterior clinoid process by tumour expansion, resulting in latero-posterior protrusion of the adenoma at the fragile dura in the oculomotor trigone. Haemorrhage may then result in sudden kinking of the oculomotor nerve at the entrance of the oculomotor trigone [12].
In our case series, radiological examinations invariably demonstrated expanding pituitary mass causing mass effect on the cavernous sinus, thereby either causing direct mechanical compression of the third cranial nerve or the vascular supply, resulting in isolated third cranial nerve palsy. All five patients suffered from partial or complete third cranial nerve palsy, with or without pupil sparing. This may represent the extent of compression or degree of vascular compromise.
The timely diagnosis and management of pituitary apoplexy is the key to a good outcome. Initial treatment should focus on hemodynamic stabilization, correction of electrolyte imbalances, and corticosteroid administration, with early consult to endocrinology and neurosurgery [31]. Due to the rarity of the condition and the lack of randomized controlled trials in the literature, the role and the timing of neurosurgical decompression remains controversial. Apart from patients with worsening neurological or ophthalmic functions, it is unclear for most patients whether conservative or surgical management carries the best outcome [32]. A few retrospective observational studies suggested early decompression to achieve better visual and endocrine outcomes [20, 21]. But several more recent retrospective studies suggested no difference in the endocrine and visual outcome between patients who were managed conservatively and patients who had early surgical intervention [19, 33, 34]. Haider [13] in 2013 reported a compilation of case reports of isolated oculomotor nerve palsy without visual deficits between 1950 and 2011 and concluded that conservative treatment can be successful in certain cases. According to Tedd et al [14], the indications for neurosurgical decompression are severely reduced visual acuity, severe and persistent/deteriorating visual field defects, or deteriorating level of consciousness.
The current consensus is that patients with significant neuro-ophthalmic signs or reduced level of consciousness should have early surgical decompression, while patient without any neuro‐ophthalmic signs or mild and stable signs can be considered for conservative management with careful monitoring [32, 35]. However, patients who are managed conservatively should be closely monitored, and surgical intervention must be considered if their condition fails to improve or show signs of deterioration. If surgical decompression is indicated, a semi-elective transsphenoidal surgery by an experienced pituitary surgeon is recommended in clinically stable patients, preferably within 7 days of the onset of symptoms [32].
Many studies have reported improvement in visual acuity, visual field defects, and ophthalmoplegia in most of the patients after surgical decompression [20-22, 36]. Such improvement is observed in the immediate postoperative period and often continues for several weeks after surgery [37]. In patients with pituitary apoplexy presenting with third cranial nerve palsy, complete recovery of the third nerve function was observed in several reports [38-41]. In our case series, complete recovery of the third nerve was achieved in 3 of the 5 cases, and partial recovery was observed in the remaining 2 cases. There was no clear correlation between time to operation with the extent of recovery, but in all 5 cases, the surgery was done within 2 weeks. We also observed that a longer time to recovery seems to be associated with incomplete (partial) recovery.
Some studies suggested that pituitary hormone deficiencies usually do not recover once established [2,42], and nearly 80 % of the patients will need some form of hormone replacement after apoplexy [21,33,34]. On the contrary, some studies have shown partial or complete recovery of pituitary function in up to 50 % of patients [43,44]. All patients with pituitary apoplexy will need long-term follow-up to monitor for recurrent tumour, and to assess their pituitary hormone levels.
Conclusion
We report a case series of pituitary apoplexy presenting as isolated left third cranial nerve palsy and a review of the possible mechanisms for this rare but potentially life-threatening medical emergency. We suggest that pituitary apoplexy should be considered early in the differential diagnosis of isolated third cranial nerve palsy and early imaging should be obtained. Once diagnosed, treatment should be commenced immediately to stabilize the patient’s hemodynamic status. The subsequent management should be made carefully by a multidisciplinary team, including neurosurgeon, endocrinologist, and ophthalmologist, amongst others.
References
- Bailey P. Pathological report of a case of acromegaly, with special reference to the lesion in the hypophysis cerebri and in the thyroid gland; and a case of haemorrhage into the pituitary. Phila Med J. 1898; 1:789–792.
- Briet C, Salenave S, Bonneville JF, Laws ER, Chanson P. (2015) Pituitary Apoplexy. Endocrine Reviews. 36(6): 622–645.
- Brougham M, Heusner AP, Adams RD (1950) Acute degenerative changes in adenomas of the pituitary body–with special reference to pituitary apoplexy. J Neurosurg. 7(5): 421– 439.
- Cardoso ER, Peterson EW (1984) Pituitary apoplexy: a review. Neurosurgery. 14(3):3 63–373
- Reid RL, Quigley ME, Yen SS (1985) Pituitary apoplexy. A review. Arch Neurol. 42(7): 712–719
- Wakai S, Fukushima T, Teramoto A, Sano K (1981) Pituitary apoplexy: its incidence and clinical significance. J Neurosurg. 55(2): 187-93.
- Sheehan HL, Stanfor JP (1961) The pathogenesis of postpartum pituitary necrosis of the anterior lobe of the pituitary gland. Acta Endocrinol 37(4): 479–510
- Molitch ME (1991) Gonadotroph-cell pituitary adenomas. N Engl J Med. 324(9): 626–627.
- Lau KK, Joshi SM, Ellamushi H, Afshar F (2007) Isolated bilateral oculomotor nerve palsy in pituitary apoplexy: case report and review. Br J Neurosurg. 21(4): 399–402
- Saul RF, Hilliker JK (1985) Third nerve palsy: the presenting sign of a pituitary adenoma in five patients and the only neurological sign in four patients. J Clin Neuroophthalmol. 5(3): 185-93.
- Cairns H. Peripheral ocular palsies from the neuro-surgical point of view. Trans Ophthalmol Soc UK 1938; 58:464 – 82.
- Kobayashi H, Kawabori M, Terasaka S, Murata J, Houkin K (2011) A possible mechanism of isolated oculomotor nerve palsy by apoplexy of pituitary adenoma without cavernous sinus invasion: a report of two cases. Acta Neurochir (Wien). 153(12): 2453-6
- Haider AS, Rao PJ (2013) A 64-year-old woman with dilated right pupil, nausea, and headache. Dijital Journal of Ophthalmology. 19(1): 13-7.
- Tedd HM, Tuckett J, Arun C, Dhar A (2012) An unusual case of sudden onset headache due to pituitary apoplexy: a case report and review of the new UK guidelines. J R Coll Physicians Edinb 42(2): 119–23
- Walsh FB (1949) Bilateral total ophthalmoplegia with adenoma of the pituitary gland: report of two cases; an anatomic study. Arch Ophthal. 42(5): 646-654.
- Cho WJ, Joo SP, Kim TS, Seo BR (2009) Pituitary apoplexy presenting as isolated third cranial nerve palsy with ptosis: two case reports. J Korean Neurosurg Soc. 45(2): 118-21.
- Rossitch E Jr, Carrazana EJ, Black PM (1992) Isolated oculomotor nerve palsy following apoplexy of a pituitary adenoma. J Neurosurg Sci. 36(2): 103-5.
- Verrees M, Arafah BM, Selman WR (2004) Pituitary tumor apoplexy: characteristics, treatment, and outcomes. Neurosurg Focus. 16(4): E6.
- Sibal L, Ball SG, Connolly V, James RA, Kane P, et al. (2004) Pituitary apoplexy: a review of clinical presentation, management, and outcome in 45 cases. Pituitary. 7(3): 157– 163.
- Bills DC, Meyer FB, Laws Jr ER, Davis DH, Ebersoldet MJ, et al. (1993) A retrospective analysis of pituitary apoplexy. Neurosurgery. 33(4): 602-608.
- Randeva HS, Schoebel J, Byrne J, Esiri M, Adams CB, et al. (1999) Classical pituitary apoplexy: clinical features, management, and outcome. Clinical Endocrinology (Oxf). 51(2): 181-8.
- Onesti ST, Wisniewski T, Post KD (1990) Clinical versus subclinical pituitary apoplexy: presentation, surgical management, and outcome in 21 patients. Neurosurgery. 26(6): 980– 986.
- Lewin IG, Mohan J, Norman PF, Gibson RA, Francis JR (1988) Pituitary apoplexy. BMJ. 297(6662): 1526–1527.
- Lloyd MH, Belchetz PE (1977) The clinical features and management of pituitary apoplexy. Postgraduate Medical Journal. 53(616): 82 – 85
- Modi P, Arsiwalla T (2021) Cranial Nerve III Palsy. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021 Jan-.
- Parkinson D. Surgical anatomy of the cavernous sinus. In: Wilkins RH, Rengachary SS, editors. Neurosurgery. New York: Mc Graw Hill; 1985. pp. 1478–1483.
- Kawase T, van Loveren H, Keller JT, Tew JM (1996) Meningeal architecture of the cavernous sinus: clinical and surgical implications. Neurosurgery 39(3): 527 – 34.
- Diyora B, Nayak N, Kukreja S, Kamble H (2011) Sudden onset isolated complete third nerve palsy due to pituitary apoplexy. Oman J Ophthalmol. 4(1): 32-34.
- Cahill M, Bannigan J, Eustace P (1996) Anatomy of the extraneural blood supply to the intracranial oculomotor nerve. Br J Ophthalmol. 80(2): 177-181.
- Kerr FW, Hollowell OW (1964) Location of pupillomotor and accommodation fibres in the oculomotor nerve: Experimental observations on paralytic mydriasis. J Neurol Neurosurg Psychiat. 1964, 27(5): 473-81.
- Salehi N, Firek A, Munir I (2018) Pituitary Apoplexy Presenting as Ophthalmoplegia and Altered Level of Consciousness without Headache. Case Rep Endocrinol. 2018: 7124364.
- Rajasekaran S, Vanderpump M, Baldeweg S, Drake W, Reddy N, et al. (2011) UK guidelines for the management of pituitary apoplexy. Clin Endocrinol (Oxf). 74(1): 9-20.– 986.
- Ayuk J, McGregor EJ, Mitchell RD, Gittoes NJL (2004) Acute management of pituitary apoplexy–surgery or conservative management? Clinical Endocrinology (Oxf). 61(6): 747–752.
- Gruber A, Clayton J, Kumar S, Robertson I, Howlett TA, et al. (2006) Pituitary apoplexy: retrospective review of 30 patients– is surgical intervention always necessary? British Journal of Neurosurgery. 20(6): 379–385.
- Capatina C, Inder W, Karavitaki N, Wass JA (2015) Management of endocrine disease: pituitary tumour apoplexy. Eur J Endocrinol. 172(5): R179-90.
- Dubuisson AS, Beckers A, Stevenaert A (2007) Classical pituitary tumour apoplexy: clinical features, management, and outcomes in a series of 24 patients. Clinical Neurology and Neurosurgery, 109(1): 63–70.
- Kerrison JB, Lynn MJ, Baer CA, Newman SA, Biousse V, et al. (2000) Stages of improvement in visual fields after pituitary tumor resection. American Journal of Ophthalmology, 130(6): 813– 820.
- Milazzo S, Toussaint P, Proust F, Touzet G, Malthieu D (1996) Ophthalmologic aspects of pituitary apoplexy. Eur J Ophthalmol. 6(1): 69–73.
- Famularo G, Pozzessere C, Piazza G, DE Simone C (2001) Abrupt-onset oculomotor paralysis: an endocrine emergency. Eur J Emerg Med. 8(3): 233–236.
- Chen Z, Murray AW, Quinlan JJ (2004) Pituitary apoplexy presenting as unilateral third cranial nerve palsy after coronary artery bypass surgery. Anesth Analg. 98(1): 46–48.
- Brisman MH, Katz G, Post KD (1996) Symptoms of pituitary apoplexy rapidly reversed with bromocriptine. Case report. J Neurosurg. 85(6): 1153 – 1155.
- Glezer A, Bronstein MD (2015) Pituitary apoplexy: pathophysiology, diagnosis, and management. Arch Endocrinol Metab. 59(3): 259-64.
- Zayour DH, Selman WR, Arafah BM (2004) Extreme elevation of intrasellar pressure in patients with pituitary tumor apoplexy: relation to pituitary function. Journal of Clinical Endocrinology and Metabolism, 89(11): 5649– 5654.
- Arafah BM, Harrington JF, Madhoun ZT, Selman WR (1990) Improvement of pituitary function after surgical decompression for pituitary tumor apoplexy. Journal of Clinical Endocrinology and Metabolism. 71(2): 323-8.