Chung Ray Ern*, Goh Chun Peng, Lim Su Lone, Ira Sun, Low Shiong Wen
Division of Neurosurgery, Department of General Surgery, Ng Teng Fong General Hospital, Singapore.
*Corresponding Author: Chung Ray Ern, Division of Neurosurgery, Department of General Surgery, Ng Teng Fong General Hospital, Singapore.
Abstract
The supplementary motor area (SMA) plays an important role in motor and speech execution. Unilateral SMA syndrome is a well-known clinical syndrome. In contrast, bilateral SMA syndrome is less commonly reported. We report a case of bilateral SMA syndrome resulting in expressive aphasia and tetraplegia following resection of parasagittal brain metastases whose speech returned to normal on post-operative day (POD) 7 and limbs power in 1 month. We discuss the clinical presentation and expected course of neurological recovery, along with their possible underlying mechanisms.
Introduction
SMA syndrome is a neurosurgical phenomenon characterized by contralateral motor deficits and speech impairment. This can manifest clinically as hemiplegia and mutism and can range from none to global akinesia. Aetiologies of SMA syndrome are mainly iatrogenic, with unilateral SMA syndrome most commonly occurring as a result of surgery involving the SMA [1,2,3,4,5]. The incidence of SMA syndrome varies greatly in published literature, with Samuel et al. describing an incidence of 23-100% following resection of the SMA [6]. Bilateral SMA syndrome is less common with few cases being reported [7]. While rare, the clinical significance of bilateral SMA syndrome is considerable due to its similarity in presentation to ‘locked-in syndrome’ which can cause distress to a patient, as well as its uniquely transient nature of symptoms, often leading to a complete functional recovery. As such, recognising bilateral SMA syndrome as a potential complication of SMA-involving surgery and counselling patients accordingly is critical especially with regards to managing patients’ expectations regarding recovery.
In neurosurgical practice, SMA syndrome is a clinical diagnosis based on a patient’s symptoms and their temporal relations to SMA- involving surgery. While the diagnosis is usually made by a neurosurgeon, a multidisciplinary approach to evaluation and treatment with other subspecialty inputs may be considered, including but not limited to radiology (specifically neuro-radiology), physiotherapy, occupational therapy, speech therapy and rehabilitation medicine etc.
The SMA-proper, corresponding to Brodmann area 6, is located in the superior frontal gyrus in the posterior aspect [10,11,12,13]. Its functions include behavioural planning, movement execution as well as speech production [14].
We present a case of bilateral SMA syndrome resulting in expressive dysphasia and tetraplegia following uncomplicated debulking of parasagittal brain metastases. Fortunately, her speech deficit resolved within 1 week and limbs power in a 1 month. During this period of ordeal, she was understandably anxious about her neurological prognosis. In this article, we conducted a literature review on the clinical presentation and course of neurological recovery of bilateral SMA syndrome, along with their possible underlying mechanisms.
Case Summary
A 33-year-old Chinese woman presented with a two-week history of progressively worsening bilateral lower limb weakness, urinary incontinence, nausea, vomiting, and neck and back pain. She had a significant medical history of Grade 2 left breast carcinoma, estrogen receptor/progesterone receptor (ER/PR) positive and human epidermal growth factor receptor 2 (HER2) negative, for which she underwent a left breast fat skin-sparing mastectomy three years earlier.
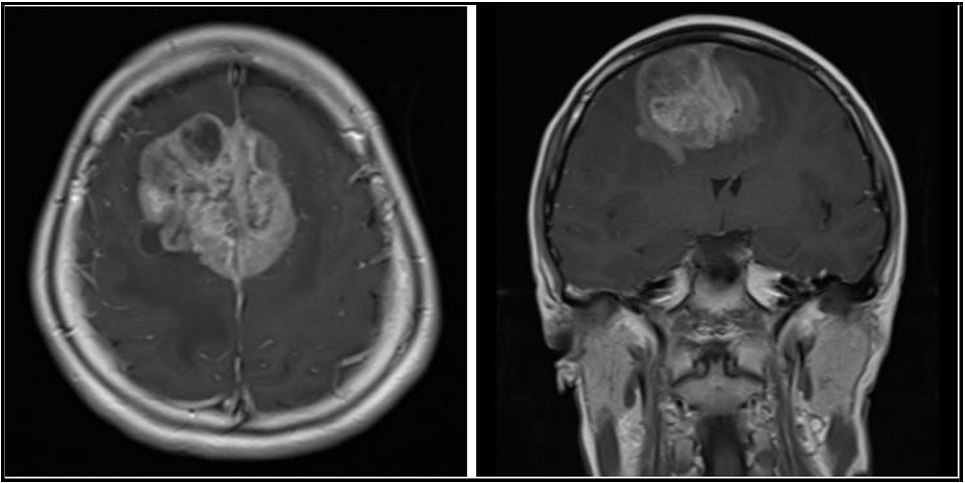
Her upper limbs power were Medical Research Council (MRC) Scale 4+ out of 5 and lower limbs power were MRC 4-/5. The rest of her neurological examination was normal. Initial non-contrast magnetic resonance imaging (MRI) of the whole spine revealed mild multilevel degenerative disc disease without significant disc bulge or protrusion. However, a subsequent cranial MRI showed a 5.2 x 5.8 x 4.1 cm enhancing heterogeneous parasagittal mass centered on the bilateral superior frontal gyrus, right larger than left, involving the region of the supplementary motor area (SMA). There was marked surrounding vasogenic edema and considerable mass effect on the bilateral lateral ventricles and corpus callosum, with a noted left midline shift (see Figure 1 for pre-operative MRI).
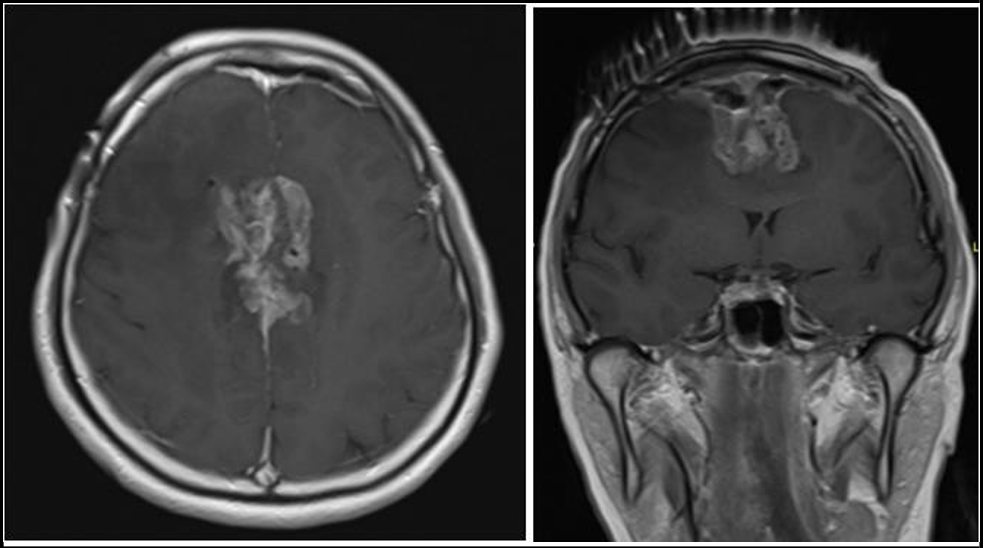
Surgical debulking was indicated due to presence of significant mass effect on the brain with perilesional oedema. The aim of surgery is to improve her quality of life and to improve the efficacy of adjuvant chemoradiation therapy. The patient underwent a craniotomy and debulking of the tumor through an interhemispheric approach. Care was taken to minimize brain retraction and debulking was performed with a combination of bipolar cautery and Cavitron Ultrasonic Surgical Aspirator (CUSA). Due to concerns about the Callos marginal arteries coursing through the tumor, further resection at the base was limited, leaving an inferior remnant (see Figure 2 for post- operative MRI). Importantly, there was no evidence of significant restricted diffusion implying ischaemia. The final histology was consistent with metastatic breast carcinoma.
Figure 1: Axial (A) and coronal (B) T1-weighted images post-gadolinium contrast enhancement MRI images show an enhancing heterogeneous parasagittal mass measuring 5.2 x 5.8 x 4.1 cm involving both cerebral hemispheres at the region of the supplementary motor areas.
Figure 2: Axial (A) and coronal (B) T1-weighted image post-gadolinium contrast enhancement MRI image shows shows interval reduction in size with grossly stable perilesional oedema post subtotal debulking of the tumour.
Postoperatively, she was tetraplegic and exhibited expressive aphasia. Over the next two days, her neurological condition improved, with initial limb power recovery observed on the left side, followed by the right. She was able to communicate using communication boards, demonstrating preserved receptive language abilities. Her speech function improved significantly and by seven days post-surgery, she was able to converse in full sentences. She underwent inpatient rehabilitation. Her limbs power recovered to MRC 5 and was discharged functionally independent on postoperative day 28.
She was started on letrozole and completed a course of stereotactic radiation therapy (30Gy over 5 fractions) to the surgical cavity and remnant tumour.
Literature Review
Reports on bilateral SMA syndrome in existing literature are limited. A keyword search with “bilateral supplementary motor area syndrome” and “bilateral SMA syndrome” was conducted on Pubmed, revealing three case reports by Heiferman et al., Friedler et al. and Wangapakul et al. [7,8,9]. We compare the aetiology, clinical presentation, onset of symptoms and duration of recovery of these cases with our patient (Table 1).
Table 1: Cases of bilateral SMA syndrome.
|
Author |
Title |
Aetiology |
Presentation |
Onset of Symptoms |
Duration of recovery |
|
Heiferman et al. (2014) |
Bilateral supplementary motor area syndrome |
Parasagittal |
Quadriparesis, |
Within 24 hours |
2 years |
|
Parasagittal |
Quadriparesis, |
1 week |
2 years |
||
|
Friedler et al. (2024) |
Bilateral Supplementary Motor Area Syndrome |
Parafalcine meningioma |
Quadriparesis, dysphasia |
Less than 72 hours |
12 weeks |
|
Wangapakul et al. (2024) |
Akinetic mutism following bilateral parasagittal meningioma occupied supplementary motor area removal and the spontaneous recovery of |
Parasagittal meningioma |
Quadriparaesis, aphasia |
24 to 48 hours |
1 year |
The aetiologies of bilateral SMA syndrome in current literature are remarkably similar, with all three case reports describing a parafalcine or parasagittal meningioma as the inciting lesion that was resected prior to the onset of the syndrome. To our knowledge, our patient is the first case of a bilateral SMA syndrome caused by a metastatic carcinoma instead of a primary brain tumour.
A key observation to note is that in all cases of bilateral SMA syndrome, the tumour is always located at the parasagittal region. This results in an SMA lesion that spans both the right and left SMA, giving rise to the characteristic syndrome. It follows therefore that most tumours described in literature are meningiomas due to the proximity of the meninges to the parasagittal area, and the growth of a parasagittal meningioma would invariably lead to bilateral involvement of the SMA. As our case report describes, brain metastases at a similar location can have similar bilateral neurological sequelae as a meningioma.
Comparing the presentations of the cases, all three authors described cases of patients who presented with profound motor deficits and patients in all three case reports developed quadriparesis, as did our patient. While patients described by Heiferman, Wangpakul and us displayed complete aphasia, the patient described by Friedler et al. did not display a similar magnitude of aphasia in their patient. The authors provided little detail of the speech deficit that their patient experienced, limiting any meaningful comparison of the type of speech deficits experienced by the patients.
The onset of symptoms described varied across the board from an acute onset post-operatively to a subacute onset of up to one week, both described by Heiferman et al. Our patient demonstrated a similar acute onset of symptoms within 24 hours while the patient described by Wangapakul was noted to have an onset of symptoms between 24 to 48 hours. Friedler et al. provided limited description of the onset of symptoms of their patient but can be inferred to have an onset of less than 72 hours.
The duration of complete recovery of the symptoms was extremely varied. Heiferman et al. described a recovery time of 2 years for both their patients in their report, while Friedler et al. and Wangpakul et al. described a recovery time of 12 weeks and 1 year respectively. By contrast, our patient had a recovery time of 4 weeks, a notably fast time of recovery compared to other case reports. This is possibly attributed to very cautious brain retraction during surgery which may have resulted in lesser damage to the SMA.
Discussion
Patients with SMA syndrome present with severe impairment of voluntary movements and aphasia, usually after ischaemia or surgery involving the SMA-proper. We discuss possible mechanisms of motor and speech deficits in SMA syndrome as well as its characteristic transient nature, comparing cases of unilateral and bilateral SMA syndrome.
Motor deficits are one of the defining clinical features of SMA syndrome. The mechanism behind motor deficits in SMA can been postulated to be due to the SMA’s structural relationship with other motor areas. The SMA is closely linked to the primary motor cortex as well as the prefrontal cortex, with some studies suggesting the SMA complex serving as a gateway between the two areas [23]. Other cortical and subcortical structures include corticospinal tracts, the primary motor, premotor and cingulate cortices, basal ganglia, cerebellum, thalamus and the contralateral SMA, as seen in existing literature [25,26,27,28,29,30]. A well-defined somatotropic organisation of the SMA is also observed with the caudal end showing a lower limb representation and the rostral end showing a face representation [24], although the relationship between this and the pattern of motor deficits seen in SMA syndrome is not well- researched.
Speech deficits in SMA syndrome commonly manifests as aphasia and mutism [18], which can be disconcerting for patients. The SMA plays a supraordinate role in speech communication [10], while the SMA-proper is thought to be involved in aspects of initiation and timing in speech processing [21] and exhibits strong connectivity to the motor cortex [22]. It is posited that the SMA-proper is linked to the language network via numerous major pathways, involving the thalamus, basal ganglia, cerebellum and premotor cortex [10]. Of note, the SMA is also linked with the Broca area, with an increased resting state connectivity between the two areas being reported in a case of auditory verbal hallucinations [31]. The patient mentioned in this case report demonstrated a difficulty in producing speech but preservation of comprehension ability consistent with expressive aphasia and was able to communicate through the use of communication boards. This phenomenon may be possibly explained by the SMA’s numerous connections with other areas of the brain including the Broca area.
A cardinal feature of SMA syndrome is the transient nature [14] of neurological symptoms. Recovery is progressive with return of function usually beginning one week after surgery with subsequent resolution of deficits within weeks to days to weeks [15,16], rarely persisting beyond one year [14]. The transient nature of neurological deficits found in unilateral SMA syndrome is believe to arise from the redundancy of the SMA proper, in which the contralateral SMA- proper assumes some function when the SMA-proper is affected by tumours or vascular malformations [17]. Neuroplasticity of the SMA- proper along with bilateral connectivity allows for reorganisation of activity in the SMA-proper, enabling functional recovery [18,19, 20]. By contrast, the recovery of bilateral SMA syndrome has been reported as being more gradual as compared to that in unilateral SMA syndrome [7]. The mechanism behind recovery in SMA syndrome is less well-known and several have been proposed, including venous hypertension and oedema following superior sagittal sinus (SSS) ligation, transient ischemia associated with post-operative vasospasm and post-operative epileptic activity within the SMA-proper, although there is insufficient literature to demonstrate these convincingly [7]. We note that the patient mentioned in this case report had near- resolution of symptoms including expressive aphasia within a month post-surgery which differs from previously reported slower recovery in other cases of bilateral SMA syndrome, suggesting that the transient deficits observed could be due to the intrinsic neuroplasticity of the SMA-proper rather than the postulated mechanisms above.
During surgery, great care was taken to avoid excessive retraction of the brain parenchyma. Despite this, she developed bilateral SMA syndrome. However, as long as there is no evidence of haemorrhage and infarction on the post-operative MRI, we can counsel patients on this syndrome which carries a favourable prognosis.
Conclusion
SMA syndrome is a neurosurgical phenomenon characterised by transient deficits in motor and speech functions with subsequent recovery. Bilateral SMA involvement is less commonly reported with variable recovery time of neurological deficits. Postoperative imaging to rule out hemorrhage or infarction is essential. Clinicians as well as patients should persevere during this challenging period while expecting recovery.
References
- Mazlan M, Fauzi AA (2011) Complete paraparesis following resection of parasagittal meningioma: recovering function with an early intensive neurorehabilitation program. Med J Malaysia. 66(4): 371-3.
- Russell SM, Kelly PJ (2003) Incidence and clinical evolution of postoperative deficits after volumetric stereotactic resection of glial neoplasms involving the supplementary motor area. Neurosurgery. 52(3): 506-16; discussion 515-6.
- Krainik A, Lehericy S, Duffau H, Capelle L, Chainay H, et al. (2003) Postoperative speech disorder after medial frontal surgery: role of the supplementary motor area. Neurology. 60(4): 587-94.
- Duffau H, Lopes M, Denvil D, Capelle L (2001) Delayed onset of the supplementary motor area syndrome after surgical resection of the mesial frontal lobe: a time course study using intraoperative mapping in an awake patient. Stereotact Funct Neurosurg. 76(2): 74-82.
- Rostomily RC, Berger MS, Ojemann GA, Lettich E (1991) Postoperative deficits and functional recovery following removal of tumors involving the dominant hemisphere supplementary motor area. J Neurosurg. 75(1): 62-8.
- Samuel N, Hanak B, Ku J, Moghaddamjou A, Mathieu F, et al. (2020) Postoperative isolated lower extremity supplementary motor area syndrome: case report and review of the literature. Childs Nerv Syst. 36(1): 189-195.
- Heiferman DM, Ackerman PD, Hayward DM, Primeau MJ, Anderson DE, et al. (2014) Bilateral supplementary motor area syndrome causing akinetic mutism following parasagittal meningioma resection. Neurosci Discov. 2(1): 7
- Friedler B, Gilbert O, Aseem F, Krawchuk L, Strohm T, et al. (2024) Bilateral Supplementary Motor Area Syndrome Presenting with Functional Quadriparesis and Apraxia. Neurology, 102(17 supplement 1): 3787.
- Wangapakul T, Kayssi AR, Riley AEM (2024) Akinetic mutism following bilateral parasagittal meningioma occupied supplementary motor area removal and the spontaneous recovery of symptoms. Surg Neurol Int. 15: 150.
- Hertrich I, Dietrich S, Ackermann H (2016) The role of the supplementary motor area for speech and language processing. Neurosci Biobehav Rev. 68: 602-610.
- Hiroshima S, Anei R, Murakami N, Kamada K (2014) Functional localization of the supplementary motor area. Neurol Med Chir (Tokyo). 54(7): 511-520.
- Orgogozo JM, Larsen B (1979) Activation of the supplementary motor area during voluntary movement in man suggests it works as a supramotor area. Science. 206(4420): 847-50.
- Penfield W, Welch K (1951) The supplementary motor area of the cerebral cortex; a clinical and experimental study. AMA Arch Neurol Psychiatry. 66(3): 289-317.
- Laplane D, Talairach J, Meininger V, Bancaud J, Orgogozo JM (1977) Clinical consequences of corticectomies involving the supplementary motor area in man. J Neurol Sci. 34(3): 301-14.
- Abel TJ, Buckley RT, Morton RP, Gabikian P, Silbergeld DL (2015) Recurrent Supplementary Motor Area Syndrome Following Repeat Brain Tumor Resection Involving Supplementary Motor Cortex. Neurosurgery. 11 Suppl 3(03): 447-55; discussion 456.
- Krainik A, Lehericy S, Duffau H, Vlaicu M, Poupon F, et al. (2001) Role of the supplementary motor area in motor deficit following medial frontal lobe surgery. Neurology. 57(5): 871– 878.
- Sailor J, Meyerand ME, Moritz CH, Fine J, Nelson L, et al. (2003) Supplementary motor area activation in patients with frontal lobe tumors and arteriovenous malformations. AJNR Am J Neuroradiol. 24(9): 1837-1842.
- Palmisciano P, Haider AS, Balasubramanian K, Dadario NB, Robertson FC, et al. (2022) Supplementary Motor Area Syndrome After Brain Tumor Surgery: A Systematic Review. World Neurosurg. 165: 160-171.e2.
- Krainik A, Duffau H, Capelle L, Cornu P, Boch AL, et al. (2004) Role of the healthy hemisphere in recovery after resection of the supplementary motor area. Neurology. 62(8): 1323-32.
- Aizawa H, Inase M, Mushiake H, Shima K, Tanji J (1991) Reorganization of activity in the supplementary motor areaassociated with motor learning and functional recovery. Exp Brain Res. 84(3): 668-71.
- Brendel B, Hertrich I, Erb M, Lindner A, Riecker A, et al. (2010) The contribution of mesiofrontal cortex to the preparation and execution of repetitive syllable productions: An fMRI study. NeuroImage. 50(3): 1219-1230.
- Wang L, Liu Q, Li H, Hu D (2013) Functional Connectivity- Based Parcellation of Human Medial Frontal Cortex via Maximum Margin Clustering, in: Yang, J., Fang, F., Sun, C. (Eds.), Intelligent Science and Intelligent Data Engineering. Springer Berlin Heidelberg. 306-312.
- Sjoberg RL (2021) Free will and neurosurgical resections of the supplementary motor area: a critical review. Acta Neurochir. 163(5): 1229-1237.
- Fried I, Katz A, McCarthy G, Sass KJ, Williamson P, et al. (1991) Functional organization of human supplementary motor cortex studied by electrical stimulation. J Neurosci. 11(11): 3656–3666.
- Maier MA, Armand J, Kirkwood PA, Yang HW, Davis JN, et al. (2002) Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: an anatomical and electrophysiological study. Cereb. Cortex. 12(3): 281–296.
- Luppino G, Matelli M, Camarda R, Rizzolatti G (1993) Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J. Comp. Neurol. 338(1): 114–140.
- Akkal D, Dum RP, Strick PL (2007) Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar output. J Neurosci. 27(40): 10659–10673.
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, et al. (2003) Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 6(7): 750-7.
- Liu J, Morel A, Wannier T, Rouiller EM (2002) Origins of callosal projections to the supplementary motor area (SMA): a direct comparison between pre-SMA and SMA-proper in macaque monkeys. J Comp Neurol. 443(1): 71-85.
- Potgieser AR, de Jong BM, Wagemakers M, Hoving EW, Groen RJ (2014) Insights from the supplementary motor area syndrome in balancing movement initiation and inhibition. Front Hum Neurosci. 8: 960.
- Clos M, Diederen KM, Meijering AL, Sommer IE, Eickhoff SB (2014) Aberrant connectivity of areas for decoding degraded speech in patients with auditory verbal hallucinations. Brain Struct Funct. 219(2): 581-94.