Nattaya Sintawichai1, Panisenee Lawasut2, Nattaya Poovorawan1, Napa Parinyanitikul1, *
1Division of Medical Oncology, Department of Medicine, Faculty of Medicine, King Chulalongkorn Memorial hospital and Chulalongkorn University
2Division of Hematology, Department of Medicine, Faculty of Medicine, King Chulalongkorn Memorial hospital and Chulalongkorn University
*Corresponding Author: Napa Parinyanitikul, Medical Oncologist, King Chulalongkorn Memorial Hospital and Chulalongkorn University, Bangkok, Thailand
Abstract
Immunotherapy has emerged as the new therapeutic frontier of cancer treatment including triple-negative breast cancer. In IMPassion130, combination atezolizumab with nab-paclitaxel demonstrated better survival outcomes compared to chemotherapy alone in metastatic TNBCs (mTNBCs) with PD-L1 positive subgroup [1]. Although immune-related adverse events (irAEs), which can affect multiple organ sites can commonly occur. However, hematologic adverse events especially autoimmune hemolytic anemia (AIHA) was rarely reported but often cause severe complications with immunotherapy treatment [2].
This clinical data reported AIHA was a rare adverse event of interest of atezolizumab in the treatment of metastatic TNBC. This event was be discussed with current knowledge and evidence of data associated with previous reports of immune‐related adverse events in using immune checkpoint inhibitors.
Keywords: autoimmune hemolytic anemia, immune-related adverse event, triple-negative breast cancer.
Introduction
Triple-negative breast cancer (TNBC) defined as negative in estrogen receptor, progesterone receptor and human epidermal growth factor receptor-2 contribute to 20 % of all breast cancer subtypes. Approximately 40 % of these patients had stage IV De NOVO disease which reported poor survival outcomes after standard chemotherapy. Median overall survival is only around 12-16 months. Therefore, various novel strategies for overcoming resistance mechanisms of this poor outcome were explored. Previous studies reported that TNBC has a high number of tumor-infiltrating lymphocytes (TILs) and PD- L1 (programmed cell death ligand 1) levels than non-TNBC [3] than other subtypes of breast cancer which make TNBC might be got benefit after using immunotherapy. Additionally, TNBC has a high level of neoantigens which can activate specific T cells to enhanced antitumor immune response [4]. Previously, immune checkpoint inhibitors (ICIs) have been investigated as monotherapy in late line setting with negative results [4]. However, currently, combination ICIs and chemotherapy has been demonstrated of great efficacy in PD-L1 positive mTNBCs, first-line setting. Atezolizumab is an IgG1 monoclonal antibody that targets PD-L1 (programmed cell death ligand 1) inhibits the interaction between PD-1 (program cell death protein 1) and B7.1 receptor leading to escaping of tumor immune response. In IMPassion130, phase 3 trial, which included and randomly assigned patients with untreated mTNBC to receive nab- paclitaxel with or without atezolizumab showed prolonged progression-free survival (PFS) from 5.0 months to 7.5 months between atezolizumab group compared to placebo (stratified hazard ratio for progression or death, 0.62; 95 % CI, 0.49 to 0.78; P<0.001) in PD-L1 positive population. The median overall survival (OS) was 25 months in atezolizumab/nab-paclitaxel group compared to 15.5 months placebo/nab-paclitaxel group (stratified hazard ratio for death, 0.62; 95 % CI, 0.45 to 0.86) [1]. The survival benefit has not been reported in the PD-L1 negative population. Hence, both the US FDA and EMA currently approved combination atezolizumab and nab-paclitaxel as the standard of care in first-line treatment of mTNBC with PD-L1 positive. In term of immune‐related adverse events, skin rash (all grades; 28.8 %, Grade 3-4; 2.3 %), hepatitis (all grades; 12 %, Grade 3-4; 4.1 %), hypothyroidism (all grades; 12.7 %, Grade 3-4; 0.3 %), hyperthyroidisms (all grades; 4.1 %, Grade 3-4; 0.3 %), pneumonitis (all grades; 2.8 %, Grade 3-4; 1.5 %) and colitis (all grades; 2.3 %, Grade 3-4; 1.3 %) were reported. No evidence of autoimmune hemolytic anemia (AIHA) as adverse events of special interest was found in this study.
AIHA characterized by acute onset of anemia accompanying with evidence of biochemical data of hemolysis and positive direct antiglobulin test (DAT). When AIHA develop after initiation or re- initiation of immunotherapy, the possibility of immune-related adverse event was suspected [3]. AIHA infrequently occurs after treating with PD-1 or PD-L1 checkpoint inhibitors approximately 0.15-0.25 % which is higher than anti-CTLA-4 (0.06 %). In other, solid cancer settings, the incidence of AIHA from atezolizumab was reported rarely 0.24 % and could occur in early or later after this drug administration. The steroid is the mainstay of AIHA treatment. Unfortunately, around 20 % of this irAE had a fatal outcome [5].
Here, we reported clinical information of metastatic triple-negative breast cancer patient treated with atezolizumab plus paclitaxel. Rare hematological immune-related adverse event, AIHA, were observed and discussed with the current knowledge of evidence from several previous studies about immune‐related adverse events after immune checkpoint inhibitors administration.
Case report
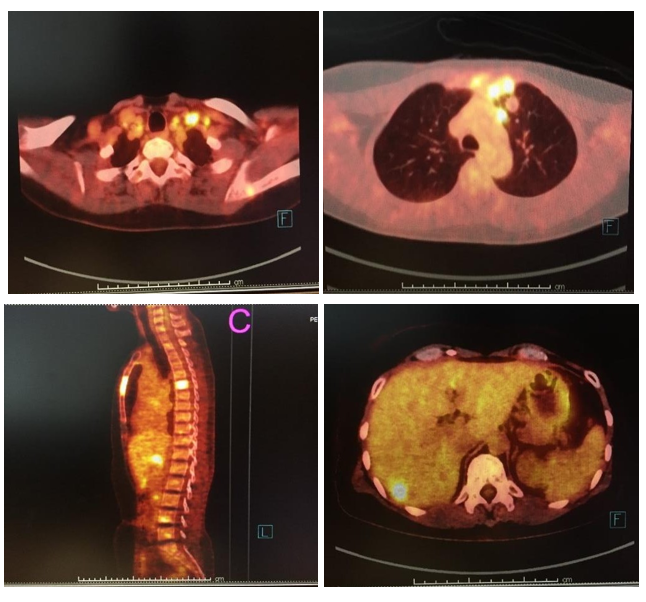
A 50-year-old female previously was diagnosed with stage II (pT2N0M0) left triple-negative breast cancer in 1999, 20 years ago. Left breast-conserving therapy with axillary lymph node dissection and completed classical CMF were given. Postoperative radiotherapy was performed after adjuvant chemotherapy. In August 2019, she developed a new left breast mass. Mammogram and ultrasound breast showed BIRAD4c. Then USG-guides FNA was performed and showed mammary ductal carcinoma. Chest and upper abdominal CT scan for staging revealed a 3.9 cm enhancing breast mass with several small satellite nodules in the left breast, a 3.6 cm matted left internal mammary lymph node, and multiple small left subpectoral, axillary, and supraclavicular lymphadenopathies. She underwent left simple mastectomy in September 2019 and pathological findings reported invasive ductal carcinoma, grade III with wide-spread lymph vascular invasion and satellite tumor (ER: 0 %, PR: 0 %, HER2-negative, Ki67: 90 %). Even with no family history of breast cancer, genetic BRCA1/2 testing was performed and showed VUS BRCA2. Radiation at the left chest wall, internal mammary lymph node, and axilla was given after palliative surgery. However, PET/CT scan showed several bones, liver, and lymph node metastases. Her PET/CT was shown in figure 1. Serum CEA and CA 15-3 were 0.96 ng/mL (0-5) and 14.66 IU/mL (0-30), respectively. Before starting the palliative chemotherapy, PD-L1 testing was recommended and PD- L1 immunohistochemistry reported positive (>10 % of immune cells). Based on the IMPassion130 study, atezolizumab plus paclitaxel, not nab-paclitaxel was given on 16th December 2019. Per our institution's practice, dexamethasone premedication was omitted in C1D15 because of no previous hypersensitivity reaction. Neither severe hematologic toxicity nor GI and neurological toxicity was observed during the first and second cycles of treatment. Additionally, no immune-related adverse events (IrAEs) were reported.
Figure 1: PET/CT scan showed A) multiple left cervical lymphadenopathies B) left internal mammary lymphadenopathies C) multiple bone metastases and D) liver metastases.
On cycle 3rd, C3D1, CBC showed anemia grade II (Hb 8.1 g/dL, Hct 25.2, RDW 19.9 %) but no neutropenia or thrombocytopenia was observed. She denied a history of blood loss either GI, GU, or elsewhere. Her anemia got worse to grade III (Hb 6.5 g/dL, Hct 21.7, RDW 22.9 %) and she had symptomatic palpitation and functional class change. So, hematology consultation was introduced. Her peripheral blood smear confirmed normochromic-normocytic anemia, polychromasia 2+, various in size of spherocytes 2+ but no schistocyte and normal NRBC/white blood cell and platelet. Further investigations showed serum ferritin 1,191 ng/mL (13-150), serum iron 38 ug/dL (37-145), TIBC 176 ug/dL (228-428), reticulocyte count 2.1 % (1-2), haptoglobin 167 mg/dL (35-250), LDH 915 U/L (140-280) and negative direct and indirect antiglobulin tests were identified. Severe autoimmune hemolytic anemia (AIHA) was the final diagnosis. Since no concomitant medications related to the etiology of AIHA were demonstrated. Immune-hemolytic anemia which is a rare immune-related adverse event from atezolizumab was suspected. Therefore, the only atezolizumab was temporarily discontinued on cycle 4th, C4D1. Due to her severe hemolysis, transfusion of leucocyte poor packed red cell and prednisolone with dose 60 mg/day (1MKD) was initiated. Summary serial CBC reports during this event was shown in table 1. Moreover, mild transaminitis, possibly from atezolizumab and paclitaxel occurred during hemolysis. Her blood testing for IrAEs was summarized in table 2. Two weeks after prednisolone, anemia with her symptoms of dyspnea and palpitation gradually improved (CBC: Hb 8.7 g/dL, Hct 28.7 %, WBC 5,300 cell/mm3, N 70.8 %, Platelet 228,000 cell/mm3). Prednisolone was then taped with a dose of 10 mg per week. In our case of grade III hemolysis and pandemic COVID-19 infection, resuming atezolizumab was not considered. Currently, palliative paclitaxel alone was then administered without additional adverse events.
Table 1: Serial CBC testing in our case report
- Before starting atezolizumab on 12/16/2019
- Before starting C2D1 on 1/20/2020
- Before starting C3D1 on 2/17/2020
- Before starting C4D1 on 3/16/2020
- Two weeks after prednisolone on 3/26/2020
- Four weeks after prednisolone on 4/9/2020
|
CBC |
12/16/2019 (A) |
1/20/2020 (B) |
2/17/2020 (C) |
3/16/2020 (D) |
3/26/2020 (E) |
4/9/2020 (F) |
Normal range |
|
RBC (X106/uL) |
4.37 |
|
2.77 |
2.27 |
3.18 |
2.94 |
3.9 - 5.5 |
|
Hb (g/dL) |
13.4 |
13.8 |
8.1 |
6.5 |
9.4 |
8.7 |
12 - 15 |
|
Hct (%) |
40.4 |
41.2 |
25.2 |
21.7 |
30.2 |
28.7 |
36 - 45 |
|
MCV (fL) |
92.4 |
91.4 |
91 |
95.6 |
95.0 |
97.6 |
80 - 100 |
|
MCH (pg) |
30.7 |
30.8 |
29.2 |
28.6 |
29.6 |
29.6 |
27 - 33 |
|
MCHC (g/dL) |
33.2 |
33.2 |
32.1 |
30.0 |
31.1 |
30.3 |
33 - 37 |
|
RDW (%) |
13.5 |
14 |
19.9 |
22.9 |
21.3 |
22.4 |
11.0-14.5 |
|
WBC (cell/mm3) |
5,100 |
3,600 |
3,690 |
4,240 |
5,660 |
5,300 |
4,500- 11,000 |
|
PMN (%) |
57.5 |
59 |
54.3 |
70 |
82.6 |
70.8 |
40-70.9 |
|
ANC (cell/mm3) |
2930 |
2,124 |
1,880 |
2,920 |
4,650 |
3,700 |
1,800-7,800 |
|
Platelet (cell/mm3) |
141,000 |
126,000 |
251,000 |
260,000 |
205,000 |
228,000 |
150,000- 450000 |
Table 2: Blood testing for IrAEs monitoring
- Before starting atezolizumab on 12/16/2019
- Before starting C3D1 on 2/17/2020
- Before starting C4D1 on 3/16/2020
|
Laboratory testing |
12/16/2019 (A) |
2/17/2020 (B) |
3/16/2020 (C) |
Normal range |
|
LFT |
|
|
|
|
|
- Total bilirubin |
0.35 |
0.51 |
0.52 |
0.2-1.2 |
|
(mg/dL) |
|
|
|
|
|
- Direct bilirubin |
0.18 |
0.15 |
0.27 |
0.00-0.50 |
|
(mg/dL) |
|
|
|
|
|
- AST (U/L) |
43 |
78 |
97 |
5-35 |
|
- ALT (U/L) |
48 |
90 |
99 |
0-40 |
|
- ALP (U/L) |
143 |
234 |
137 |
40-120 |
|
- Albumin (g/dL) |
4.5 |
|
3.8 |
3.5-5.0 |
|
Serum amylase (U/L) |
|
149 |
145 |
25-125 |
|
Serum lipase (U/L) |
|
18 |
20 |
8-78 |
|
Thyroid |
|
|
|
|
|
function test |
|
|
|
|
|
- Free T3 (ng/dL) |
2.71 |
1.68 |
0.8-1.8 |
|
|
- Free T4 (pg/mL) |
1.7 |
1.85 |
1.6-4 |
|
|
- TSH (uIU/mL) |
2.7 |
2.59 |
0.3-4.1 |
Discussions
Here, we reported AIHA with DAT negative (Grade 3; ATCAE version 5) after atezolizumab and paclitaxel administration for 8 weeks. By excluding all possibilities of abrupt onset of anemia such as drug related AIHA, irAE from atezolizumab was the likely cause of this immune hemolysis.
The mechanism of AIHA associated with immunotherapy has not been yet established. A similar hypothesis from primary AIHA was demonstrated [6,7]. However, few studies reported after ICIs were initiated, the aberrant in the host immune system has arisen. Unlike drug-induced AIHA, random activation of T immune cell impacts on the formation of autoantibodies, activation of T-cell clones, and diminishing the function of regulatory T cells [5]. DAT negative was reported in approximately 10-38 % in ICIs related AIHA. Limitation of a standard method for detecting RBC-bound IgG in which below the ability threshold or low affinity of IgG or another type of Ig such as IgA or IgM might explain the possible reasons for negative testing [8].
According to previous studies, irAEs such as skin rash, colitis, endocrinopathy, and liver toxicity after ICIs typically occurred between 4 and 6 weeks, 4 and 10 weeks, 6 and 14 weeks, and 6 and 14 weeks, respectively [9]. About the onset of atezolizumab related AIHA, our case reported the onset of anemia started within 42 days after initiation of atezolizumab which like the median interval from ICIs initiation to development of AIHA in previous data was 55 days (range 22-110 days) [3]. Comparing with other drug induced AIHA, the onset varied depends on the type of drugs which was ranging from 5 minutes to several weeks [10].
In term of treatment, our patient did show a good response after glucocorticoids with 0.5-1mg/kg/day of oral prednisolone administration. Finally, oral prednisolone was taped with gradually increased hemoglobin level within 2 weeks. Systemic corticosteroids are considered as standard first-line treatment of AIHA which are approximately 80 % effective. In the case of refractory AIHA, splenectomy, rituximab, and other immunosuppressive drugs such as azathioprine, cyclophosphamide was considered as second-line treatment options. Additional therapy, for example, intravenous immunoglobulin, plasma exchange, danazol or high-dose cyclophosphamide, and alemtuzumab can be one of the optional treatment modalities [11]. There is no different treatment between ICIs induced AIHA and drug induced AIHA. Not only aggressive treatment with high-dose corticosteroid but also intensive supportive treatment including transfusions and volume replacement is recommended in severe hemolysis.
About re-challenging ICIs, nearly 15 % of patients will develop irAEs again after re-initiate the same immunotherapy. According to previous grade III hemolysis, treatment with atezolizumab was permanently discontinued [3]. According to rare irAE, little is known about the association of AIHA and treatment outcomes especially in mTNBC.
Conclusion
AIHA associated with atezolizumab is infrequent but can develop serious immune-related adverse event leading to treatment temporally or permanently discontinuation. Early recognition and early management are essentially recommended.
References
- Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, et al. (2018) Atezolizumab and nab-paclitaxel in advanced triple- negative breast cancer. New England Journal of Medicine. 379(22): 2108-2121.
- Leaf RK, Ferreri C, Rangachari D, Mier J, Witteles W, et al. (2019) Clinical and laboratory features of autoimmune hemolytic anemia associated with immune checkpoint inhibitors. American journal of hematology. 94(5): 563-574.
- Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, et al. (2014) PD-L1 expression in triple-negative breast cancer. Cancer immunology research. 2(4): 361-70.
- Keenan TE, Tolaney SM (2020) Role of Immunotherapy in Triple-Negative Breast Cancer. Journal of the National Comprehensive Cancer Network. 18(4): 479-489.
- Tanios G, Doley PB, Munker R (2018) Autoimmune Hemolytic Anemia and Checkpoint Inhibitors: 68 Cases from the FDA Database and Critical Review. Blood. 132(Supplement 1):2324.
- Barros MM, Blajchman MA, Bordin JO (2010) Warm autoimmune hemolytic anemia: recent progress in understanding the immunobiology and the treatment. Transfusion medicine reviews. 24(3): 195-210.
- Salama A (2009) Drug-induced immune hemolytic anemia. Expert opinion on drug safety. 8(1):73-9.
- Segel GB, Lichtman MA (2014) Direct antiglobulin (“Coombs”) test-negative autoimmune hemolytic anemia: a review. Blood Cells, Molecules, and Diseases. 52(4): 152-60.
- Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, et al. (2019) Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 16(9): 563-580.
- Garratty G (2010) Immune hemolytic anemia associated with drug therapy. Blood reviews. 24(4-5): 143-50.
- Zanella A, Barcellini W (2014) Treatment of autoimmune hemolytic anemias. Haematologica. 99(10): 1547-54.