Ilaria Baglivo1*, Stefania Colantuono2, Marianna Criscuolo3, Sabrina Giammarco3, Antonio Gasbarrini1, Clara Di Mario4, Elisa Gremese4, Francesco Macagno5, Pier-Valerio Mari6, Livio Pagano3 & Cristiano Caruso2
1Department of Gastroenterology, CEMAD, Center for Diagnosis and Treatment of Digestive Diseases, Fondazione Policlinico Universitario "A. Gemelli" IRCCS, Catholic University of Rome, 00168 Rome, Italy.
2UOSD DH Internal Medicine and Gastroenterology, Fondazione Policlinico Universitario "A. Gemelli" IRCCS, Catholic University of Rome, 00168 Rome, Italy.
3Department of Radiological and Hematological Sciences, Fondazione Policlinico Universitario "A. Gemelli" IRCCS, Catholic University of Rome, 00168 Rome, Italy.
4Division of Clinical Immunology, Fondazione Policlinico Universitario “A. Gemelli” IRCCS, Catholic University of Rome, 00168 Rome, Italy.
5Division of Pulmonary Medicine, Fondazione Policlinico Universitario "A. Gemelli" IRCCS, Catholic University of Rome, 00168 Rome, Italy.
6Division of Internal Medicine, San Carlo di Nancy Hospital, GVM Care and Research, Rome, Italy.
*Corresponding Author: Ilaria Baglivo, Department of Gastroenterology, CEMAD, Center for Diagnosis and Treatment of Digestive Diseases, Fondazione Policlinico Universitario "A. Gemelli" IRCCS, Catholic University of Rome, 00168 Rome, Italy.
Abstract
Treatment with Mepolizumab has been shown to improve systemic involvement in a patient affected by systemic mastocytosis and severe eosinophilic asthma.
Eosinophil modulation affects mast cells and could be considered a therapeutic target in cases of mastocytosis unresponsive to conventional therapies.
Keywords: Mast cells; systemic mastocytosis; interleukin-5; Mepolizumab; severe eosinophilic asthma.
To the Editor,
Systemic Mastocytosis (SM) is characterized by a clonal proliferation and accumulation of multifocal clusters of abnormal Mast Cells (MCs) in one or more tissues or organ systems.
SM includes both indolent and aggressive forms. In MC disorders, the clinical symptoms are attributable to the release of MC mediators or the direct tissue infiltration and could involve skin, bone, gastrointestinal, cardiovascular, respiratory, and neurologic systems. Although, in recent years, new drugs have been studied for the treatment of MC disorders, the therapeutic approach, especially in the case of Aggressive Systemic Mastocytosis (ASM), remains a clinical challenge.
It is known that eosinophils and MCs interact [1]. In the last decade, the employment of anti-eosinophils Monoclonal Antibodies (mAbs) has modified both clinical practice and outcomes in eosinophilic disorders; no evidence is currently available about the effect of these drugs on MCs.
We describe the case of a 64-year-old woman diagnosed with severe eosinophilic asthma in 2007. Treatment with high doses of Inhaled Corticosteroids (ICS), Long-acting Beta Agonists (LABA), and Long-Acting Muscarinic Antagonists (LAMA) was established.
Gastroesophageal reflux disease, spinocerebellar ataxia, hepatic steatosis, and hypertension were comorbidities.
In 2008, the patient underwent frontal osteoma removal surgery, and in 2009, she presented multiple vertebral fractures, some of which required vertebroplasty.
2016, the patient presented dyspnea, asthenia, arthralgia, itching, flushing, urticaria, and diarrhea. Blood tests showed increased eosinophil count (340 cell/mm3) and serum tryptase (20.7 µg/l). The patient underwent Bone Marrow Biopsy (BMB), indicating the presence of atypical CD25pos/CD2posMCs multifocal compact aggregates with paratrabecular and interstitial distributions. Although the D816V cKIT mutation was undetectable, the patient was diagnosed with ASM, according to the 2016 WHO criteria [2]. Therapy with Oral Corticosteroids (OCS) (prednisone 25 mg/die) and antihistamines were started. However, constitutional symptoms were poorly controlled, so the patient underwent different cytoreductive treatments: from March 2017 to August 2018, interferon, dasatinib, and finally, cladribine were consecutively employed as therapeutic approaches, but they were all stopped due to intolerance or ineffectiveness.
Although a subsequent BMB, performed in 2018, showed histological remission, serum tryptase was persistently increased (>20 µg/l), and constitutional symptoms were still uncontrolled and negatively impacted the patient's quality of life.
Worsening of dyspnea was the primary cause of frequent emergency room accesses and high dose OCS pulses, so, according to severe eosinophilic asthma diagnosis, therapy with anti-IL-5 mAb (mepolizumab 100 mg every 4 weeks) was started on June 2022.
After 6 months of therapy, the patient showed clinically and functional relevant improvement. After treatment, Forced Expiratory Volume in the first second (FEV1) was 86% versus 73% pre-therapy; Fractional Exhaled Nitric Oxide (FeNO) improved from 31 ppb pre-therapy to 18 ppb, no further OCS pulse was needed.
Moreover, improvements in general, gastrointestinal, and cutaneous symptoms were observed: asthenia and arthralgia gradually disappeared, itching resolved, and no further episodes of flushing or diarrhea occurred. Serum tryptase decreased to 4.6 µg/l, and blood eosinophil count was 30 cells/mm3. These findings have also been confirmed at 12 months of clinical evaluation.
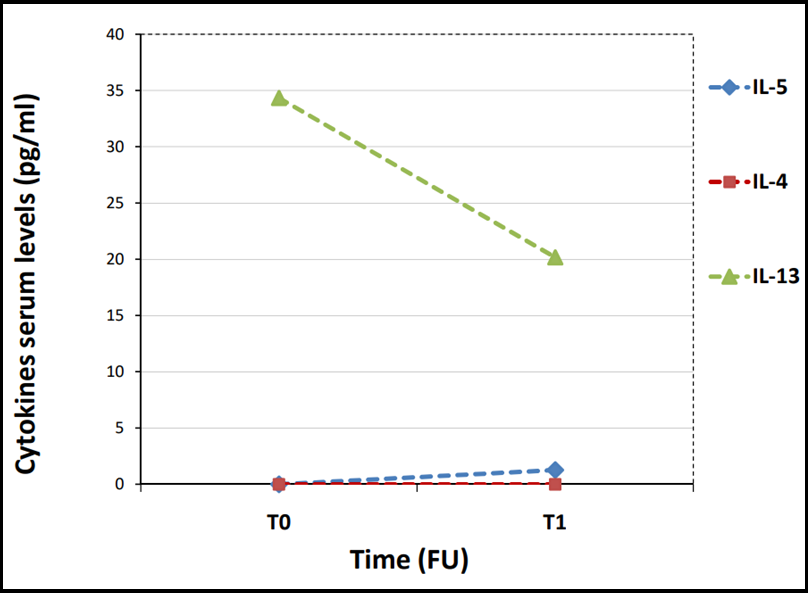
Type 2 (T2) inflammatory cytokines were measured before mepolizumab treatment (4 weeks after the last OCS administration) (T0) and after 6 months of therapy (T1), using Enzyme-Linked Immunosorbent Assay (ELISA) kits for serum IL-5, IL-4, IL-13 (Bio-Techne, UK). Analyses were performed according to the manufacturer's instructions for each kit. The minimum detectable dose was 1.08 pg/mL for IL-5, 10 pg/ml for IL-4, and 3.46 pg/ml for IL-13.
At T0, the serum levels of IL-5 were undetectable (<1.08 pg/ml), as well as IL-4 (<10 pg/ml), whereas serum IL-13 was 34.33 pg/ml. At T1, serum IL-13 levels decreased to 20.17 pg/ml, whereas IL-4 serum levels remained undetectable. Moreover, IL-5 serum levels slightly increased to 1.27 pg/ml during treatment. (Figure 1) shows the trend of the cytokines levels during mepolizumab treatment.
Figure 1: Type 2 cytokines serum levels at baseline (T0) and after 6 months of mepolizumab treatment (T1).
Although the BMB resulted in histological remission after cytoreductive treatments, tryptasemia persisted, and clinical symptoms were poorly controlled. The therapy with anti-IL-5 mAb was shown to improve both clinical manifestations and laboratory findings.
The inhibition of IL-5 could modulate MCs through direct or eosinophil-mediated mechanisms. In T2 inflammation, MCs release IL-5, promoting eosinophil survival, differentiation, and activation. Moreover, an autocrine effect of IL-5 on MCs was suggested by Ochi et al., who showed that IL-5 could have a priming effect on MCs, modulating their phenotype and increasing the production of a defined profile of cytokines [3].
MCs/eosinophils cross-stimulation is allowed by different mechanisms involving surface receptors; it has been reported that MCs and eosinophils are found in couplets in the nasal mucosal tissue of patients with allergic rhinitis [4].
Only one similar case has been described in the literature so far. Guillet et al. [5], in 2020, reported an idiopathic MC activation syndrome who experienced an excellent clinical and laboratory response to mepolizumab administered according to a severe asthma schedule.
Moreover, mepolizumab decreased the number of esophageal tryptase-positive MCs in pediatric patients affected by eosinophilic esophagitis [6].
The role of cytokines in MC diseases is well known. MCs secrete a broad spectrum of cytokines with pleiotropic effects.
In our case, we evaluated the modification of T2 cytokines serum concentration after mepolizumab treatment, and we observed a reduction of IL-13 after 6 months of treatment. It is well known that MCs synthesize IL-13 upon stimulation. In T2 response, IL-13 seems to have a more prominent role in the peripheral tissues, such as airway remodeling, while IL-4 appears more relevant for central response, such as in lymph nodes [7].
Moreover, we observed a slight increase of IL-5 serum levels during treatment, compared to baseline, despite improvement in asthma and ASM symptoms and blood eosinophil decrease. This data could be influenced by mepolizumab/IL-5 complex interference. As previously reported, serum IL-5 appears to not correlate with clinical response [8].
In conclusion, our data highlight that MCs/eosinophils and MCs/IL-5 interactions could have an essential role in developing and maintaining MC diseases, and IL-13 could be actively involved.
Further studies are needed to investigate new potential therapeutic targets in MC diseases. The possible efficacy of anti-IL-5 drugs, as shown in this case, may represent a topic of speculation.
Abbreviations
Systemic Mastocytosis (SM); Mast Cells (MCs);
Aggressive Systemic Mastocytosis (ASM);
Monoclonal Antibodies (mAbs);
Long-acting Beta Agonist (LABA);
Long-Acting Muscarinic Antagonist (LAMA);
Bone Marrow Biopsy (BMB);
Oral Corticosteroids (OCS);
Forced Expiratory Volume in the first second (FEV1);
Fractional Exhaled Nitric Oxide (FeNO);
Enzyme-Linked Immunosorbent Assay (ELISA); Type 2 (T2).
Consent for Publication
Informed consent was obtained from the patient to publish the case report and all accompanying visual elements.
Conflict of interests
The authors declare that they have no conflict of interest.
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
MC, SG, and LP contributed substantially to the acquisition, analysis, and interpretation of data and the hematological evaluation of the patient. IB played a significant role in the writing and design of the manuscript. CC, SC, CDM, and EG analyzed and interpreted the immunological data of the patient. FM and PVM contributed to the pneumological evaluation of the patient. SC, AG, and CC revised the manuscript critically for important intellectual content and gave final approval for publishing the version. All authors have read and approved the final manuscript, agree to be accountable for all aspects of the work, and will ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data availability
The data supporting this study's findings are available from the corresponding author upon reasonable request.
References
- Elishmereni M, Bachelet I, Nissim Ben-Efraim AH, Mankuta D, Levi-Schaffer F (2013) Interacting mast cells and eosinophils acquire an enhanced activation state in vitro. Allergy. 68(2): 171- 9.
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, et al. (2016) The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 127(20): 2391-405.
- Ochi H, De Jesus NH, Hsieh FH, Austen KF, Boyce JA (2000) IL- 4 and -5 prime human mast cells for different profiles of IgE- dependent cytokine production. Proc Natl Acad Sci USA. 97(19): 10509-13.
- Elishmereni M, Alenius HT, Bradding P, Mizrahi S, Shikotra A, et al. (2011) Physical interactions between mast cells and eosinophils: a novel mechanism enhancing eosinophil survival in vitro. Allergy. 66(3): 376-85.
- Guillet C, Steinmann S, Lang C, Maul JT, Schmid-Grendelmeier P (2021) Eosinophil-mast cell interaction: Mepolizumab leads to a reduction of clinical symptoms and serum tryptase in a patient with eosinophilic asthma and idiopathic mast cell activation. J Allergy Clin Immunol Pract. 9(3): 1393-1395.e1.
- Otani IM, Anilkumar AA, Newbury RO, Bhagat M, Beppu LY, et al. (2013) Anti-IL-5 therapy reduces mast cell and IL-9 cell numbers in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol. 131(6): 1576-82.
- Bao K, Reinhardt RL (2015) The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine. 75(1): 25- 37.
- Caruso C, Colantuono S, Tolusso B, Di Mario C, Pentassuglia A, et al. (2021) Basophil activation and serum IL-5 levels as possible monitor biomarkers in severe eosinophilic asthma patients treated with anti-IL-5 drugs. Allergy. 76(5): 1569-1571.