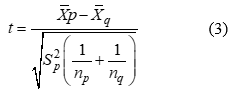Usman Muhammad Tukur1*, Shamsuddeen Idris Musa2*, Zainab Aliyu Adam2, Bishir Usman3, Rabi Rabi’u Abubakar4, Sani Rabi’u5 , Sunday Alaku Ibbih6, Muhammad Adam Abubakar7, Ni’imatullahi Kabir Isah8
1School of Metallurgy and Environment, Central South University, Changsha, China
2General Studies, Emirates College of Health, Kano, Kano State, Nigeria
3Department of Pure and Industrial Chemistry, Bayero University Kano, Kano State, Nigeria
4Science Laboratory Technology, Federal Polytechnic, Kazaure, Jigawa State, Nigeria
5General Studies, School of Health Technology, Daura, Katsina State, Nigeria
6Department of Community Health, Nasarawa State University, Keffi, Nasarawa State, Nigeria
7Department of Actuarial Science, Federal University Dutse, Jigawa State, Nigeria
8Kano Agricultural Supply Company, Kano State, Nigeria
Corresponding Author: Usman Muhammad Tukur & Shamsuddeen Idris Musa, School of Metallurgy and Environment, Central South University, Changsha, China & General Studies, Emirates College of Health, Kano, Nigeria
Abstract
Many years ago, periodontal disease precisely posed significant oral health challenges, resulting in complications such as tooth mobility and tooth loss. This condition demonstrates a strong association with several systemic diseases, particularly diabetes mellitus. The relationship is substantial as periodontal disease can enhance insulin resistance, thereby complicating diabetes management. This study examined the treatment response rates in patients with simultaneous periodontal disease and diabetes, while also investigating the statistical correlation between these conditions. To derive meaningful insights, the research utilized secondary data spanning a decade (2015-2024) from the dental clinics in Kano State. The research tested two hypotheses, Null Hypothesis (H0): Patients with concurrent diabetic and periodontal diseases do not exhibit accelerated treatment response. Alternative Hypothesis (H1): Patients with periodontal disease demonstrate rapid treatment response. Statistical analysis, conducted using SPSS software, employed correlation analysis and t-tests to examine the relationship between periodontal and diabetic treatments (variables X1 and X2). The results revealed a robust correlation coefficient (r) of 95.7% between the variables. Additionally, t-test results demonstrated statistical significance, confirming a strong linear relationship between the treatment modalities for both conditions. Based on these findings, the study highlights the interconnected nature of periodontal and diabetic treatments. The research recommends that healthcare providers implement specialized treatment protocols for diabetic patients with periodontal complications. This integrated approach is essential for optimizing treatment outcomes and effectively managing the complex interplay between these conditions.
Keywords: Oral health, Periodontal, Diabetic, Treatments, Healthcare, Analysis
1. Introduction
Periodontal disease, commonly referred to as gum disease, represents a chronic inflammatory condition that poses a significant threat to oral health. This progressive infection targets the gums and can lead to devastating effects on both soft tissue and the underlying bone structure that provides essential support for teeth [1-2]. The condition stands as one of the two most critical challenges to oral health worldwide and serves as the primary factor in tooth loss among adults [3]. The development of periodontal disease is intricately linked to inadequate oral hygiene practices, which create favorable conditions for plaque accumulation. This plaque manifests as a transparent, adhesive biofilm containing numerous bacteria that adheres to the tooth surface [4]. When left undisturbed, this bacterial film triggers an inflammatory response in the surrounding gum tissues, initiating the disease process [5].
The complexity of periodontal disease is highlighted by the remarkable diversity of the oral microbiome, with scientific research identifying approximately 800 distinct bacterial species residing within the oral cavity. The pathogenesis of periodontal disease is not simply a matter of bacterial presence alone, but rather involves a sophisticated interplay between bacterial infection and the host's immune response. This interaction is further complicated by various behavioral and environmental factors, with smoking being a particularly significant modifier of disease progression and treatment outcomes [6-7].
Periodontal diseases encompass a spectrum of conditions that affect the supporting structures of teeth, including the gums, periodontal ligaments, and alveolar bone. These supporting tissues play crucial roles in maintaining dental health and stability [8]. Without appropriate intervention, the disease process can lead to a cascade of destructive events, including the deterioration of gum tissue, degradation of the alveolar bone (the specialized region of the jawbone that houses the teeth), and damage to the cementum (the outer layer of the tooth root) [9]. The condition manifests in several distinct forms and progresses through various stages, each with its own characteristic features and severity levels. These range from mild gingivitis, which is reversible with proper care, to severe periodontitis, which can cause irreversible damage to oral tissues. Understanding these different stages is crucial for proper diagnosis and treatment planning. The impact of periodontal disease extends beyond oral health, as research has increasingly demonstrated links between periodontal inflammation and various systemic health conditions. This understanding has elevated the importance of periodontal disease prevention and treatment in the context of overall health maintenance and disease prevention [10].
These Include:
Gingivitis, recognized as the earliest and mildest form of periodontal disease, is a reversible inflammatory condition affecting the gum tissue surrounding the teeth. It arises primarily due to the accumulation of bacterial plaque—a sticky, colorless biofilm—along the gumline and between teeth. When oral hygiene practices such as brushing and flossing are inconsistent or inadequate, plaque builds up, creating an environment where harmful bacteria thrive. These microbes release toxins that irritate the gingiva (gum tissue), triggering the body’s immune response. This inflammatory reaction is the hallmark of gingivitis and serves as the body’s attempt to combat bacterial invasion. However, if not addressed promptly, this localized inflammation can escalate into more severe forms of periodontal disease. The most common indicators of gingivitis include visible redness and swelling of the gums, which may appear puffy or overly tender [11]. Healthy gums typically exhibit a pale pink hue and firm texture, but inflamed gingiva often take on a darker red or purplish tint. A key symptom is bleeding during routine activities such as brushing, flossing, or even light probing during dental examinations. This bleeding occurs because the inflamed gum tissue becomes fragile and prone to rupture under minor pressure. While discomfort or pain is not always present, some individuals may experience sensitivity or a persistent metallic taste due to blood in the mouth. Importantly, gingivitis is often overlooked because its early symptoms can be mild or intermittent, leading many to delay seeking care until the condition worsens [12].
Figure 1: Early stage of periodontal disease.
Periodontitis represents the severe progression of untreated gingivitis, marking a critical escalation in periodontal disease where inflammation extends beyond superficial gum tissue to infiltrate the deeper structures supporting the teeth. This irreversible stage is characterized by the destruction of the alveolar bone (the jawbone that houses tooth sockets), periodontal ligaments (connective tissues anchoring teeth to bone), and cementum (the protective outer layer of tooth roots). As the infection advances, the gums begin to detach from the teeth, forming pathological spaces known as periodontal pockets. These pockets become reservoirs for harmful bacteria, plaque, and tartar, accelerating tissue degradation and creating a self-sustaining cycle of inflammation and damage. The transition from gingivitis to periodontitis occurs when persistent bacterial toxins and the body’s hyperactive immune response overwhelm the gingiva’s ability to heal. Immune cells release inflammatory mediators, such as cytokines and enzymes like collagenase, which inadvertently break down connective tissues and bone. As the alveolar bone resorbs and gum tissue recedes, teeth lose their structural support, leading to mobility, shifting, or misalignment. Deepening pockets—often exceeding 4 millimeters in depth—harbor anaerobic bacteria that thrive in low- oxygen environments, exacerbating infection [13].
Figure 2: Untreated periodontal disease.
Advanced Periodontitis: In the most severe stage, the supporting structures of the teeth are severely damaged, leading to significant tooth loss. Treatment at this stage may involve surgical procedures and more intensive dental care [14].
Figure 3: Severe periodontal disease.
The current global statistics from 2025 indicate that, periodontal disease affects more than one billion people worldwide, with severe cases found in 743 million individuals, representing 11% of the global population [15-17]. The impact is especially noticeable in developing nations, where inadequate access to dental healthcare services worsens the problem [18]. The widespread occurrence of periodontal disease across age groups has elevated it to a major public health issue. Risk factors encompass lifestyle choices (smoking), oral hygiene practices, medical conditions (diabetes), medication use, age-related changes, genetic factors, and stress levels [19-21]. Scientific evidence strongly links periodontal disease to broader health issues, including heart disease, diabetes, and pregnancy-related complications [22]. The severity of this connection is highlighted by the fact that Type 2 diabetic patients with advanced periodontal disease have a mortality risk more than three times higher than those with minimal or no periodontitis [23-24]. Importantly, managing periodontal health has been shown to enhance glycemic control in Type 2 diabetes patients [25-27].
Figure 4: Oral Health Management Strategy.
Although oral health research has made considerable strides, critical gaps persist in developing effective global treatment strategies [28- 30]. Key challenges include complex healthcare referral pathways, prohibitive treatment expenses, systemic disease complications, and limited comprehension of how periodontal and diabetic treatments interact in patients with both conditions [31-34].
Here, the study implemented a dual analytical approach, combining treatment response analysis in patients with concurrent periodontal disease and diabetes with statistical correlation studies using secondary data. Three main objectives guided the research: examining the connection between diabetes and periodontal conditions, assessing their linear relationship, and evaluating treatment response speeds in affected patients. The research outcomes suggest this methodology could lead to improved treatment optimization and better management of the complex interactions between these conditions, advancing future oral health interventions.
2.Methodology
The data used in this research work is secondary data from 2015 to 2024, which was collected from the Dental clinics in Kano State.
2.1 Hypothesis
Ho: patients with diabetic and periodontal diseases do not respond to treatment quickly.
H1: patients with periodontal disease only respond to treatment quickly.
2.2 Statistical Tools Used in the Analysis
2.2.1 Correlation
Let us consider two variables x and y. The correlation R is given by equation 1 below.
By analogy, the deviation from the mean 




2.3. To Test for the Significance of Correlation
A test for the statistical significance of correlation can be carried out using T-test. Thus, we proceed,
Hypothesis:
Ho: Patients with diabetic and periodontal diseases do not respond to the treatment quickly.
H1: Patients with periodontal diseases only respond to the treatment quickly.
Level of significance 
Test statistic using equation 3 below:
Where 

Reject the null hypothesis, if 
We can reject the null hypothesis, if
3. Results and Discussion
The research findings of this investigation are illustrated in the subsequent tables and figures, revealing the strong interrelationship between treatments for periodontal disease and diabetes.
Table 1: Patients with periodontal and diabetic diseases over the years.
|
Year |
2015 |
2016 |
2017 |
2018 |
2019 |
2020 |
2021 |
2022 |
2023 |
2024 |
|
Periodontal disease(X1) |
170 |
164 |
132 |
162 |
174 |
168 |
178 |
200 |
164 |
102 |
|
Diabetic disease (Y1) |
16 |
18 |
22 |
28 |
27 |
31 |
38 |
44 |
40 |
32 |
Table 2: Treatments of patients with periodontal and diabetic diseases over years.
|
Year |
2015 |
2016 |
2017 |
2018 |
2019 |
2020 |
2021 |
2022 |
2023 |
2024 |
|
Periodontal treatment(X2) |
126 |
122 |
102 |
120 |
141 |
144 |
138 |
174 |
131 |
78 |
|
Diabetic treatment(Y2) |
10 |
12 |
15 |
17 |
20 |
22 |
26 |
32 |
31 |
18 |
Table 1 and Figure 5 presents longitudinal raw data on patients registered for diagnosis in the clinic with Periodontal Disease (X1) and Diabetic Disease (Y2), highlighting that a significant number of individuals have experienced complications from both conditions over an extended period (10 years).
Figure 5: Number of cases over ten (10) years.
Figure 6: Treatments over ten (10) years
Table 2 and Figure 6, in contrast, illustrates the subset of these patients who experienced combined treatment (X2: periodontal treatment, Y2: diabetic treatment) and subsequently achieved improved health outcomes. The data in Table 2 is derived from the original patient cohort documented in Table 1, emphasizing the effectiveness of dual-treatment interventions for those with comorbid diagnoses.
Table 3: Descriptive Statistics.
|
Variable |
Mean |
Std Dev. |
Std Err |
N |
|
X1 |
161.40 |
26.767 |
8.465 |
10 |
|
Y1 |
29.600 |
9.3120 |
2.945 |
10 |
|
X2 |
127.60 |
25.665 |
8.116 |
10 |
|
Y2 |
20.300 |
7.4990 |
2.371 |
10 |
Correlation Results for Periodontal, Diabetic Disease and Periodontal, Diabetic Treatment:
Set 1 Range = Sheet1!$A$2:$C$12
Set 2 Range = Sheet1!$D$2:$F$12
The header indicates that this is a correlation, with Range 1 variables (X1, Y1) (Periodontal Disease and Diabetic disease) located at A2:C12, to be correlated with Range 2 variables X2, Y1 (Periodontal Treatment and Diabetic Treatment) located at D2:F12. The descriptive statistics table gives the mean, standard deviation, standard error and sample sizes for each of the Set 1 variables (X1, Y1) and Set 2 variables (X2, Y2). See Table 3 above.
Table 4: Simple Correlation.
|
|
|
N |
Correlation |
Sig. |
|
Pair 1 |
Periodontal Disease & Diabetic Disease (X1 &Y1) |
10 |
.282 |
.431 |
The correlation gives the simple correlation coefficients (r) for each of the X1 and Y1 variable. R is >= -1 and <= +1 for any variable correlated with any other variable. In this analysis, r is 0.282 for the simple correlation of X1 with Y1 meaning that there is a Week positive correlation between the two variables i.e., Periodontal Disease and Diabetic Disease (X1, Y1). See Table 4 above.
Table 5: Paired Samples Correlations.
|
|
|
N |
Correlation |
Sig. |
|
Pair 1 |
Periodontal Treatment (X2) & diabetic Treatment (Y2) |
10 |
.577 |
.080 |
The correlation gives the simple correlation coefficients (r) for each of the X2 and Y2 variables. R is >= -1 and <= +1 for any variable correlated with any other variable. In this analysis, r is 0.577 for the simple correlation of X2 with Y2 meaning that there is a positive correlation between the two variables i.e. Periodontal Treatment and Diabetic Treatment (X2, Y2). See Table 5 above.
Table 6: Paired Samples Correlations.
|
|
|
N |
Correlation |
Sig. |
|
Pair 1 |
Periodontal Disease(X1) WITH Periodontal Treatment (X2) |
10 |
.957 |
.000 |
The correlation gives the simple correlation coefficients (r) for each of the X1 and X2 variable. R is >= -1 and <= +1 for any variable correlated with any other variable. In this analysis, r is 0.957 for the simple correlation of X1 with X2 meaning that there is a strong positive correlation between the two variables i.e. Periodontal Disease and Diabetic Treatment (X1, X2). See Table 6 above.
Table 7: Paired Samples Correlations.
|
|
|
N |
Correlation |
Sig. |
|
Pair 1 |
Diabetic Disease (Y1) WITH Diabetic Treatment (Y2) |
10 |
.966 |
.000 |
The correlation gives the simple correlation coefficients (r) for each of the Y1 and Y2 variable. R is >= -1 and <= +1 for any variable correlated with any other variable. In this analysis, r is 0.957 for the simple correlation of Y1 with Y2 meaning that there is a strong positive correlation between the two variables i. e Diabetic Disease and Diabetic Treatment (Y1, Y2). See Table 7.
Computation: By using SPSS, we have the following output:
Table 8: Paired Sample Test
|
Paired Samples Test |
|||||||||
|
|
|
Paired Differences |
t |
df |
Sig. (2 tailed) |
||||
|
|
|
Mean |
Std. Deviation |
Std. Error Mean |
5% Confidence Interval of the Difference |
|
|||
|
Lower |
Upper |
||||||||
|
Pair 1 |
Treatment Periodontal – Treatment diabetic |
107.300 |
22.196 |
7.019 |
106.847 |
107.753 |
15.287 |
9 |
.000 |
T-TEST PAIRS=Treatment Periodontal WITH Treatment Diabetic (PAIRED)/CRITERIA=CI
(.050)/MISSING=LISTWISE.
Since the sig value 0.00 < α = 0.05, we reject Ho and conclude that the treatment patient with periodontal differ significantly with patient with both periodontal and diabetic.
Figure 7: Oral Health Management Strategy.
4. Conclusion
Conclusively, this study highlights the critical need for accurate diagnosis of periodontal disease in diabetic patients by analyzing clinical traits observed during treatment, while emphasizing the interdependence of periodontal and diabetes management. Findings reveal that diagnosing periodontal disease in diabetic populations requires meticulous care and tailored treatment protocols. Statistical analyses, correlation and t-test over multiple years demonstrated a strong relationship between periodontal disease (X1) and its treatment (X2), with a correlation coefficient confirming their simultaneous delays in treatment efficacy observed in some dental clinics in Kano. The correlation strength was statistically significant (95.7%), validating a near-absolute association between these variables. The study advocates for policymakers, researchers, and healthcare; providers to leverage these insights for advancing interdisciplinary research, refining clinical guidelines, and raising public awareness about the bidirectional risks of periodontal disease and diabetes. Targeted health education is recommended to mitigate societal and medical impacts, emphasizing preventive care and early intervention for at-risk populations.
References
- Albandar JM, Streckfus CF, Adesanya MR, Winn DM (2000) Cigarette smoking as risk factors for periodontal disease and tooth loss. Journal of Periodontal. 71(12): 1874–81.
- Albandar JM (2002) Global risk factors and risk indicators for periodontal diseases. Periodontal. 29(1): 177–206.
- Armitage GC (1999) Development of a classification system for periodontal diseases and conditions. Ann. Periodontal. 4(1): 1-6.
- Ashby MT, Kreth J, Soundarajan M, Sivuilu LS (2009) Influence of a model human defensive peroxidase system on oral streptococcal antagonism. Microbiology. 155(11): 3691–700.
- Axelsson P, Lindhe J, Nyström B (1991) On the prevention of caries and periodontal disease. Results of a 15-year longitudinal study in adults. Journal of Clinical Periodontal. 18(3): 182–9.
- Aydin S (2007) A comparison of ghrelin, glucose, alpha-amylase and protein levels in saliva from diabetics. Journal of Biochemistry and Molecular Biology. 40(1): 29–35.
- Bascones-Martínez A, Muñoz-Corcuera M, Bascones-Ilundain J (2015) Diabetes and periodontitis: A bidirectional relationship Med Clin (Barc). 145(1): 31–35.
- Benjamin RM (2010) Oral health: The silent epidemic. Public Health Rep. 125(2): 158–9.
- Bergstrom J (2014) Smoking rate and periodontal disease prevalence: 40-year trends in Sweden, 1970-2010. Journal of Clinical Periodontal. 41(10): 952–7.
- Bridges RB, Anderson JW, Saxe SR, Gregory K, Bridges SR (1996) Periodontal status of diabetic and non-diabetic men: effects of smoking, glycemic control, and socioeconomic factors. Journal of Clinical Periodontol. 67(11): 1185-1192.
- Casanova L, Hughes FJ, Preshaw PM (2014) Diabetes and periodontal disease: A two-way relationship. Br Dent J. 217(8): 433–7.
- Caton JG (2018) A new classification scheme for periodontal and peri-implant diseases and conditions Introduction and key changes from the 1999 classification. Journal of Clinical Periodontology.
- Cefalu WT (2001) Insulin resistance: cellular and clinical concepts. Exp Biol Med. 226(1): 13-26.
- Chapple IL, Genco R (2013) Working group 2 of the joint EFP/AAP workshop. Diabetes and periodontal diseases: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol. 84(4): 106– 12.
- Dandona P, Thusu K, Cook S, Snyder B, Makowski J, et al. (1996) Oxidative damage to DNA in diabetes mellitus. The Lancet. 347(8999): 444–445.
- Pablo P, Chapple IL, Buckley CD, Dietrich T (2009) Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol. 5(1): 218–24.
- Dean L, Entyre J (2004) The Genetic Landscape of Diabetes. Bethesda (MD): National Center for Biotechnology Information (US); Chapter 2, Genetic Factors in Type 1 Diabetes.
- Defronzo RA, Tripathy D (2009) Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diabetes Care. 32(2): 157–163.
- El-Shinnawi U, Soory M (2013) Associations between periodontitis and systemic inflammatory diseases: response to treatment. Recent Pat EndocrMetab Immune Drug Discov. 7(3): 169-188.
- Engebretson SP, Hey-Hadavi J (2011) Sub-antimicrobial Doxycycline for Periodontitis Reduces Hemoglobin A1c in Subjects with Type II Diabetes: A Pilot Study. Pharmacological research: the official journal of the Italian Pharmacological Society. 64(6): 624-629.
- Engebretson SP, Hey-Hadavi J, Ehrhardt FJ, Hsu D, Celenti RS, et al. (2004) Gingival crevicular fluid levels of interleukin-1beta and glycemic control in patients with chronic periodontitis and Type II diabetes. J Periodontol.75(9): 1203-1208.
- Faria-Almeida R, Navarro A, Bascones A (2006) Clinical and metabolic changes after conventional treatment of Type II diabetic patients with chronic periodontitis. Journal Periodontol.77(4): 591-598.
- Fenesy KE (1998) Periodontal disease: An overview for physicians. Mt Sinai J Med. 65(6): 362–9.
- Festa A, D'Agostino R Jr, Howard G, Mykkänen L, Tracy RP, et al. (2000) Chronic subclinical inflammation as part of the insulin resistance syndrome: The Insulin Resistance Atherosclerosis Study (IRAS). Circulation. 102(1): 42-47.
- Figuero E, Sánchez-Beltrán M, Cuesta-Frechoso S, Tejerina JM, del Castro JA, et al. (2011) Detection of periodontal bacteria in atheromatous plaque by nested polymerase chain reaction. J Periodontol. 82(10): 1469–1477.
- Firatli E (1997) The relationship between clinical periodontal status and insulin-dependent diabetes mellitus. Results after 5 years. J Periodontol. 68(2): 136-140.
- Fröhlich M, Imhof A, Berg G, Hutchinson WL, Pepys MB, et al. (2000) Association between C-reactive protein and features of the metabolic syndrome: a population-based study. Diabetes Care. 23(12): 1835-1839.
- Gabbe SG (2017) Diabetes mellitus complicating normal pregnancy. In: Obstetrics: Normal and Problem Pregnancies. 7th ed. Philadelphia, Pa.
- Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y (2005) A proposed model linking inflammation to obesity, diabetes, and periodontal infections. Journal Periodontol. 76(11): 2075-2084.
- Gonçalves LS, Gonçalves BM, Fontes TV (2013) Periodontal disease in HIV-infected adults in the HAART era: Clinical, immunological, and microbiological aspects. Arch Oral Biol. 58(10): 1385–1396.
- Grodstein F, Colditz GA, Stampfer MJ (1996) Post-menopausal hormone uses and tooth loss: A prospective study. J Am Dent Assoc. 127(3): 370–7.
- Grossi SG, Skrepcinski FB, DeCaro T, Robertson DC, Ho AW, et al. (1997) Treatment of periodontal disease in diabetics reduces glycated hemoglobin. Journal of Periodontol. 68(8): 713-719.
- Grossman E, Messerli FH (2008) Hypertension and diabetes. Adv Cardiol. 45(1): 82-106.
- Grover HS, Luthra S (2013) Molecular mechanisms involved in the bidirectional relationship between diabetes mellitus and periodontal disease. Journal of Indian Society of Periodontology. 17(3): 292–301.