Robert G. Audet, BS1,2,4, Aaron J. Tabor, PhD, CTBS, CWCA1,3,4#, Robert B. Diller, PhD, CWCA2,5, Robert S. Kellar, PhD2,3,6
1Carmell Corporation, Pittsburg PA, USA
2Axolotl Biologix Inc, Department of Research and Development, Flagstaff, AZ, USA*
Axolotl Biologix Inc, Department of Clinical Operations, Flagstaff, AZ, USA*
4Northern Arizona University, Biological Sciences, Flagstaff, AZ, USA
5Present Address: Amnio Technology, LLC, Phoenix, AZ
6Northern Arizona University, Biological Sciences and Center for Materials Interfaces in Research & Applications (¡MIRA!), Flagstaff, AZ
*prior to merger with Carmell Corporation
#Corresponding Author: Aaron J. Tabor, PhD, CTBS, Carmell Corporation, Pittsburg PA, USA, Axolotl Biologix Inc, Department of Clinical Operations, Flagstaff, AZ, USA*, Northern Arizona University, Biological Sciences, Flagstaff, AZ, USA
Abstract
Chronic skin wounds, often caused by underlying health conditions such as diabetes, obesity and malnutrition are often stalled in the inflammation stage and are unable to progress through all phases of wound healing. Current standard of care is insufficient to resolve many chronic wounds, leading to increased risk of infection and comorbidities. Regenerative therapies such as amniotic membrane and amnion cell-derived fluid, have been used to promote successful chronic wound healing and repair. In this case report, a combination of a conditioned media from human amnion-derived cells and dehydrated amniotic membrane were used in combination to treat a ten month, chronically open wound. Several applications of these two regenerative therapies in the wound resulted in the formation of granulation tissue within 7 days, restoration of normal blood flow and pain sensation within five weeks, wound closure within four months and resolution in 11.5 months. Conditioned media from human amnion-derived cells and dehydrated amniotic membrane facilitated the rapid closure and resolution of a chronic open wound.
Keywords: Chronic wound, amniotic, regenerative, biologics/stem cells
Introduction
Chronic wounds are classified as wounds arrested in the inflammatory phase of healing that remain open for > 1 month [1] or ≥ 3 months [2- 4] and negatively impact morbidity, mortality, and quality of life [5- 9]. Chronic, physiologically impaired wounds include but are not limited to diabetic foot ulcers, venous ulcers, pressure sores, and post- surgical wounds. Their refractory nature is often related to underlying complications, e.g., infection, diabetes, obesity, pressure, and venous insufficiency [1,9-11]. Armstrong et al. report that the 5-year mortality rate of persons with diabetic foot complications (DFU, Charcot foot arthropathy, minor and major amputations) was comparable to the rate for all cancer at 31 % [12]. Based on the US Medicare 5 % 2014 dataset, an estimated 8.2 million Medicare recipients are impacted by chronic nonhealing wounds, with an estimated outpatient cost of $ 9.9 – $ 35.8 billion [13]. Several treatments have been designed to mitigate the growing challenges to patients with chronic wounds and the exploding costs. The increasing number of people with chronic wounds, as well as comorbidities such as obesity, diabetes, malnutrition, peripheral arterial or venous disease, etc., require a multifactorial approach [14,15]
Standard of care (SOC) is the first approach to treating chronic wounds and includes debridement, antimicrobial treatment, traditional and synthetic wound dressing, negative pressure therapy, offloading, compression [10,16,17] and addressing the underlying causes to support the body in healing the wound naturally. However, these approaches are often insufficient to allow resolution of chronic wounds with complicated etiologies. There is an increase in clinical research incorporating a range of regenerative medicine interventions for treating chronic injuries, including growth factors [18-20], stem cells [21], placental-derived tissues [22-27], biomimetic membranes and tissue-engineered skin, ECM scaffolds 28–32, and stem cell secretome [33-35].
Allogeneic amniotic membrane has been used successfully in conjunction with SOC for treating diabetic foot ulcers, chronic vein ulcers [22-27,36,37], and chronic nonhealing wounds in elderly patients [38]. Amniotic cell-derived conditioned media has been used to investigate the mechanisms that promote the healing of chronic wounds [39,40] and reduce apoptosis in a murine model of thermal burn injuries [41].
Cell culture media is conditioned by the amniotic cells, which release the cellular secretome (soluble and insoluble factors) into the media during normal growth. This acellular media is collected, analyzed, and processed for various applications.
Materials and Methods
For this case study, conditioned media from human amnion-derived cells (CM, Axolotl Ambient™, Axolotl Biologix, Phoenix, AZ) and an a decellularized, dehydrated, human amniotic membrane (ddhAM, Axolotl DualGraft™, Axolotl Biologix, Flagstaff, AZ) were used throughout the treatment regimen. This case study was conducted in compliance with the ethical principles in the Declaration of Helsinki. Informed patient consent was obtained by the physician prior to the start of treatment.
At the time of treatment, the CM (Axolotl Ambient™) was marketed under section 361 of the Public Health Service (PHS Act and the regulations in 21 CFR Part 1271 as a Human Cells, Tissues and Cellular and Tissue-Based Products (HCT/P) within the Food, Drug Administration 42. Since the end of the FDA's discretionary enforcement period (5/31/2021), the Ambient™ CM was removed from the market and is currently in clinical trials under an FDA IND for the treatment of osteoarthritis of the ankle (NCT05092646, clinicaltrials.gov).
Conditioned Media and Acellular Human Amniotic Membrane:
The human amniotic membrane was isolated from the placenta of a scheduled cesarean section delivery and tested for infectious agents (HIV, Hepatitis B, Hepatitis C, CMV, WNV) and microbial contamination. The amnion was cleaned, washed, decellularized, folded into a dual layer with the epithelial side out, then dehydrated. The membrane was packaged, terminally irradiated, and stored at a controlled room temperature (15-30°C).
Following enzymatic decellularization, the pluripotent amniotic stromal cells released from the amniotic membrane were expanded in tissue culture flasks in media. The cell conditioned regenerative fluid was collected at specified time points, packaged, terminally irradiated, then stored at room temperature (18-32°C)
Case Study
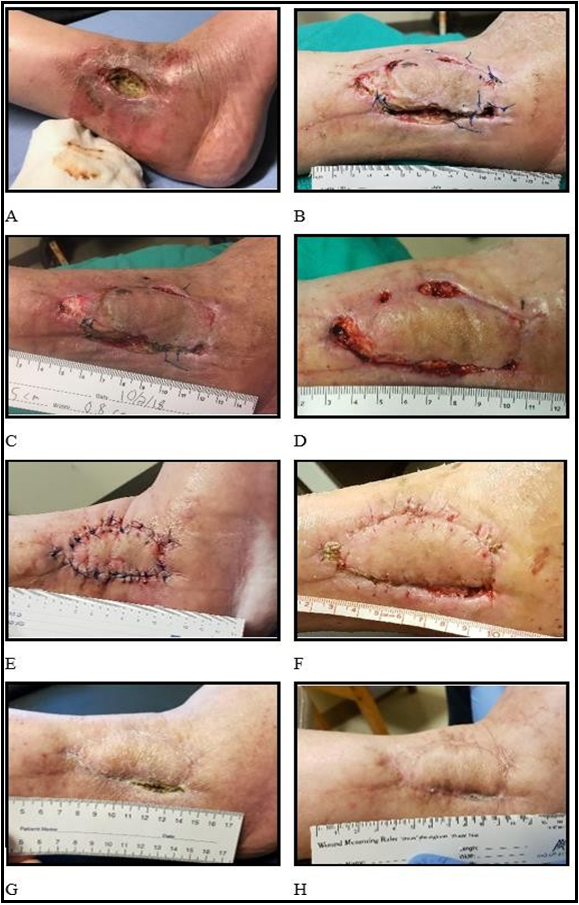
A 47-year-old female with confounding factors, including surgery-related infection, alcoholism, pre-diabetes, and compliance issues, developed a chronic wound following a fracture from a fall at home and post-surgical infection over ten months. The patient fell at her residence, resulting in a compound ankle fracture. She did not receive any intervention for three days. The fracture was surgically treated, and hardware was placed to stabilize the fracture. Ten months post-surgery, the patient presented with a chronic wound resulting from an underlying hardware infection. The hardware was surgically removed, and the patient was placed on an antibiotic regimen and referred to a plastic surgeon specializing in wound care (Figure 1A). One week later, incisions were created anterior to the wound to facilitate healing by relieving tension on the wound. Sutures were placed where the tissue could be approximated, with the remaining areas left open. Three weeks following releasing the incision, the patient began receiving treatment with 2 mL CM injected into the wound margins and within the wound bed, and a total of 32 cm2 of dHAM was cut into pieces and packed into the open areas that could not be approximated. The physician could palpate the bone through the wound opening, demonstrating minimal bleeding following debridement. The patient also reported no pain and minimal sensation. The area was covered with an Xeroform bandage as SOC (Figure 1B).
At one week follow-up, day 7, there was a visible increase in granulation tissue, several sutures were removed, and the physician could no longer palpate the bone. Following debridement, the wound was treated with 2 mL CM and 32 cm2 of dHAM and covered with Xeroform (Figure 1C). On day 16, the patient was ill with a stomach virus. Additional sutures were removed, and the wound was debrided and treated with 2 mL CM and 32 cm2 dHAM (picture not shown). After removing the infected hardware, the patient also completed the course of antibiotics prescribed. At five weeks, day 35, the wound exhibited increased blood flow at debridement and a return of pain sensation. Following debridement, the damage was treated with 2 mL CM and 16 cm2 of dHAM (Figure 1D). By the eighth week, day 53, the underlying tissue had re-grown sufficiently to approximate the wound edges, and since the patient experienced increased pain, the wound edges were surgically sutured and stapled closed under general anesthesia (Figure 1E). During follow-up at three, eight, fifteen- and twenty-three days post-surgery, the wound was treated with 2 mL CM in the wound margins, covered with Xeroform, and the ankle was splinted to limit actions. Four weeks post-surgery, day 88 post initial treatment, sutures and staples were removed, the wound was washed with normal saline, and the margins were injected with 2 mL CM and covered with Xeroform. The patient was told to begin regular foot and ankle activity (Figure 1F). At four months, day 128 from the start of treatment, the wound was closed with only a tiny portion of a sanguineous crust (scab) remaining (Figure 1G). The scab was debrided, the wound margins were injected with 2 mL CM, and the area was covered with Xeroform. At 11 months following initial treatment, day 352, the wound was fully closed (Figure 1H).
A total of ten treatments with CM and four treatments with ddhAM were administered over the four-month treatment regimen. It is likely the wound was closed in under 11 months, however, the patient did not return visit to the physician’s office for a follow up until 11 months.
Figure 1: Healing progression in chronic wounds treated with CM and dHAM. All days listed are post-initial treatment (Day 0). A: Post-surgery to remove infected hardware 34 days before the start of treatment. B: Day 0 start of treatment with CM and dHAM, following surgical incision. C: Day 7, showing notable granulation tissue in all open areas. D: Day 35, following 3 treatments with CM and dHAM. E: Day 53, following surgical suturing. F: Day 88, approximately 5 weeks from recent surgery. G: Day 128, a closed structural barrier (scab), four months from first treatment. H: Final image demonstrating a fully closed wound, Day 352.
Discussion
In developing countries, the prevalence of chronic nonhealing wounds is nearly 2.5 %. In the United States, 8.2 million Medicare patients reported at least one chronic damage, ranging from diabetic-related infections, foot, pressure, and venous ulcers to surgical conditions [13,43]. Lower extremity amputation is required in 14- 24% of patients with a diabetic foot ulcer [15]. Current population trends suggest that in 2060, there will be 77 million elderly individuals. Given the increasing population age, the incidence of chronic wounds is expected to continue to increase [13], negatively impacting the quality of life, morbidity, and mortality and placing an estimated $28-96 billion burden on the healthcare industry [7].
The chronicity of nonhealing wounds is driven by numerous comorbidities including but not limited to diet, diabetes, hypertension, renal disease 3 peripheral arterial or venous disease, [14,15], depression [5], and alcohol consumption [44]. In this case report, alcohol may be a key player in the persistence of the chronic wound. Alcohol appears to have a bimodal immunomodulatory effect, with acute consumption reducing proinflammatory cytokines and regular consumption prolonging inflammatory responses in immune cells [45]. Pre-clinical data also supports the impact of the inflammatory phase of wound healing with chronic alcohol intake. In mice, chronic alcohol increases the release of proinflammatory cytokines and a detrimental inflammatory systemic response [44].
In this case study, a 47-year-old patient with attendant alcohol consumption, pre-diabetes, and compliance challenges developed a chronic wound over 10 months following surgical intervention to address a fracture. The treatment used in this case report is a combination of SOC with two regenerative therapies: a human, acellular amniotic membrane that provides a physical structure or barrier function and a regenerative fluid secretome from multipotent, human amnion-derived stem cells derived from the amnion. Amniotic membrane (dHAM) is immune privileged and contains various growth factors and cytokines, cell adhesion molecules, matrix remodeling factors, antimicrobial peptides, and an extracellular matrix [46–49]. Conditioned media from human amnion-derived cells (CM) also contains growth factors and cytokines, cell adhesion molecules, soluble extracellular matrix proteins, and antimicrobial peptides [33,35,50]. Both treatments supply growth factors and cytokines with immunomodulatory, angiogenic, proliferative, anti-fibrotic, anti-inflammatory, and antimicrobial properties that facilitate and support the healing cascade.
The chronic alcohol consumption during the intervention may have contributed to this patient's total wound healing time. The combination of regenerative dHAM and CM provided the chronic wound with factors that interrupted the regular inflammatory cycle and progressed the injury through the proliferation phase to the maturation/remodeling phase of wound healing. Given the significant overlap of the 4 stages of wound healing and the complex interplay of innate immunity and resident skin cells, restoring the natural balance of growth factors and signaling molecules is critical for wound repair, closure, and resolution. Even with the initial stalled wound healing response and multiple comorbidities, the multiple applications of regenerative therapies demonstrated a positive clinical outcome for this patient. Future studies should include a Randomized controlled clinical study testing the regenerative fluid and ddhAM against SOC.
Key points
In this case study, a middle-aged female patient with pre-diabetes and active alcoholism developed an infection ten months after surgical intervention to address a fractured ankle. The physician created incisions surrounding the wound to relieve wound tension and administered ten treatments of allogeneic decellularized, dehydrated amniotic membrane and/or amnion cell-conditioned media regenerative therapies within and around the wound bed over the course of four months. Normal blood flow and pain sensation returned during the treatment course, at four months the wound was nearly closed with a small scab, and at eleven months the wound was closed. The growth factors and cytokines in amnion-derived therapies facilitated the progression of the chronic wound to closure amidst several health confounds and compliance issues.
Acknowledgments
We thank Dr. Oren Tessler, MD, for wound care and clinical application of products and Jon Canyon for coordinating product availability and clinical visits.
Funding: The Axolotl DualGraft™ and Axolotl Ambient™ products were provided at no cost to the physician for use in the patient.
Conflict of Interest disclosure statement
Robert Audet, Aaron Tabor, Robert Diller, and Robert Kellar have been or are currently employed by or contracted with Axolotl Biologix.
References
- Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, et al. (2009) Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair and Regeneration. 17(6): 763-71.
- Martin P, Nunan R (2015) Cellular and molecular mechanisms of repair in acute and chronic wound healing. British Journal of Dermatology. 173(2): 370-8.
- Veličković VM, Spelman T, Clark M, Probst S, Armstrong DG, et al. (2023) Individualized Risk Prediction for Improved Chronic Wound Management. Adv Wound Care (New Rochelle). 12(7): 387-398.
- Pang C, Ibrahim A, Bulstrode NW, Ferretti P (2017) An overview of the therapeutic potential of regenerative medicine in cutaneous wound healing. Int Wound J. 14(3): 450-459.
- Fino P, Di Taranto G, Pierro A, Kacjulite J, Codolini L, et al. (2019) Depression risk among patients with chronic wounds. Eur Rev Med Pharmacol Sci. 23(10): 4310-4312.
- Kapp S, Santamaria N (2017) The financial and quality-of-life cost to patients living with a chronic wound in the community. Int Wound J. 14(6): 1108-1119.
- Sen CK (2019) Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv Wound Care (New Rochelle). 8(2): 39-48.
- Shukla VK, Shukla D, Tripathi AK, Agrawal S, Tiwary SK, et al. (2008) Results of a one-day, descriptive study of quality of life in patients with chronic wounds. Ostomy Wound Management. 54(5): 43-9.
- Turner NJ, Badylak SF (2015) The Use of Biologic Scaffolds in the Treatment of Chronic Nonhealing Wounds. Adv Wound Care (New Rochelle). 4(8): 490-500.
- Borda LJ, Macquhae FE, Kirsner RS (2016) Wound Dressings: A Comprehensive Review. Curr Dermatol Rep. 5(4): 287-297.
- Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, et al. (2008) Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol. 58(2): 185-206.
- Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV et al. (2020) Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 13(1): 16.
- Sen CK (2021) Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv Wound Care (New Rochelle). 10(5): 281-292.
- Mustoe TA, O’Shaughnessy K, Kloeters O (2006) Chronic wound pathogenesis and current treatment strategies: A unifying hypothesis. Plast Reconstr Surg. 117(7 Suppl): 35S-41S.
- Tresierra-Ayala MÁ, García Rojas A (2017) Association between peripheral arterial disease and diabetic foot ulcers in patients with diabetes mellitus type 2. Medicina Universitaria. 19(76): 123-126.
- Falanga V, Isseroff RR, Soulika AM, Romanelli M, Margolis D, et al. (2022) Chronic wounds. Nat Rev Dis Primers. 8(1): 50.
- Powers JG, Higham C, Broussard K, Phillips TJ (2016) Wound healing and treating wounds Chronic wound care and management. J Am Acad Dermatol. 74(4): 607-625.
- Barrientos S, Brem H, Stojadinovic O, Tomic-Canic M (2014) Clinical application of growth factors and cytokines in wound healing. Wound Repair and Regeneration. 22(5): 569-578.
- Mast BA, Schultz GS (1996) Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair and Regeneration. 4(4): 411-420.
- Vågesjö E, Öhnstedt E, Mortier A, Lofton H, Huss F, et al. (2018) Accelerated wound healing in mice by on-site production and delivery of CXCL12 by transformed lactic acid bacteria. Proc Natl Acad Sci U S A. 115(8): 1895-1900.
- Larson A, Gallicchio VS (2017) Amniotic Derived Stem Cells: Role and Function in Regenerative Medicine. Journal of Cell Science & Therapy. 8(1): 3.
- Abdo RJ (2016) Treatment of Diabetic Foot Ulcers with Dehydrated Amniotic Membrane Allograft: A Prospective Case Series. J Wound Care. 25(Sup7): S4-S9.
- Barr SM (2014) Dehydrated Amniotic Membrane Allograft for Treatment of Chronic Leg Ulcers in Patients With Multiple Comorbidities: A Case Series. Journal of the American College of Clinical Wound Specialists. 6(3): 38-45.
- Riordan NH, George BA, Chandler TB, McKenna RW (2015) Case report of non-healing surgical wound treated with dehydrated human amniotic membrane. J Transl Med. 13: 242.
- Rosenblum BI (2016) A Retrospective Case Series of a Dehydrated Amniotic Membrane Allograft for Treatment of Unresolved Diabetic Foot Ulcers. J Am Podiatr Med Assoc.
- Ilic D, Vicovac L, Nikolic M, Lazic Ilic E (2016) Human amniotic membrane grafts in therapy of chronic non-healing wounds. Br Med Bull. 117(1): 59-67.
- Sheikh ES, Sheikh ES, Fetterolf DE (2014) Use of dehydrated human amniotic membrane allografts to promote healing in patients with refractory non healing wounds. Int Wound J. 11(6): 711-717.
- Falanga V, Sabolinski M (1999) A bilayered living skin construct (APLIGRAF®) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair and Regeneration. 7(4): 201-207.
- Marston WA, Hanft J, Norwood P, Pollak R (2003) The Efficacy and Safety of Dermagraft in Improving the Healing of Chronic Diabetic Foot Ulcers Results of a Prospective Randomized Trial. 26(6): 1701-1705.
- Mostow EN, Haraway GD, Dalsing M, Hodde JP, King D (2005) Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: A randomized clinical trial. J Vasc Surg. 41(5): 837-843.
- Rodriguez IA, Strombergsson A, Weinstein R, Maloney A, Hendrix C, et al. (2020) Preliminary Clinical Evaluation Using a Novel Bioengineered Wound Product to Treat Lower Extremity Ulcers. International Journal of Lower Extremity Wounds. 22(1): 139-145.
- Towler MA, Rush EW, Richardson MK, Williams CL (2018) Randomized, Prospective, Blinded-Enrollment, Head-To-Head Venous Leg Ulcer Healing Trial Comparing Living, Bioengineered Skin Graft Substitute (Apligraf) with Living, Cryopreserved, Human Skin Allograft (TheraSkin). Clin Podiatr Med Surg. 35(3): 357-365.
- Ahangar P, Mills SJ, Cowin AJ (2020) Mesenchymal stem cell secretome as an emerging cell-free alternative for improving wound repair. Int J Mol Sci. 21(19): 7038.
- Saheli M, Bayat M, Ganji R, Hendudari F, Kheirjou R, et al. (2020) Human mesenchymal stem cells-conditioned medium improves diabetic wound healing mainly through modulating fibroblast behaviors. Arch Dermatol Res. 312(5): 325-336.
- Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R (2017) Mesenchymal stem cell secretome: Toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 18(9): 1852.
- Serena TE, Yaakov R, Moore S, Cole W, Coe S, et al. (2020) A randomized controlled clinical trial of a hypothermically stored amniotic membrane for use in diabetic foot ulcers. J Comp Eff Res. 9(1): 23-34.
- Thompson P, Hanson DS, Langemo D, Anderson J (2019) Comparing Human Amniotic Allograft and Standard Wound Care When Using Total Contact Casting in the Treatment of Patients with Diabetic Foot Ulcers. Adv Skin Wound Care. 32(6): 272- 277.
- Regulski M (2018) Utilization of a Viable Human Amnion Membrane Allograft in Elderly Patients With Chronic Lower Extremity Wounds of Various Etiologies. Wounds. 30(3): E36- E40.
- Tang Z, Tan J, Yuan X, Zhou Q, Yuan Z, et al. (2020) Circular RNA ABCB10 promotes angiogenesis induced by conditioned medium from human amnion derived mesenchymal stem cells via the microRNA 29b 3p/vascular endothelial growth factor A axis. Exp Ther Med. 20(3): 2021-2030.
- Wang B, Lee WY, Huang B, Zhang JF, Wu T, et al. (2016) Secretome of human fetal mesenchymal stem cell ameliorates replicative senescen. Stem Cells Dev. 25(22): 1755-1766.
- Li JY, Ren KK, Zhang WJ, Xiao L, Wu HY, et al. (2019) Human amniotic mesenchymal stem cells and their paracrine factors promote wound healing by inhibiting heat stress-induced skin cell apoptosis and enhancing their proliferation through activating PI3K/AKT signaling pathway. Stem Cell Res Ther. 10(1): 247.
- Food and Drug Administration (2001) Human Cells, Tissues, and Cellular and Tissue-Based Products.
- Nussbaum SR, Carter MJ, Fife CE, DaVanzo J, Haught R, et al. (2018) An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value in Health. 21(1): 27-32.
- Malherbe DC, Messaoudi I (2022) Transcriptional and Epigenetic Regulation of Monocyte and Macrophage Dysfunction by Chronic Alcohol Consumption. Front Immunol. 13: 911951.
- Avishai E, Yeghiazaryan K, Golubnitschaja O (2017) Impaired wound healing: Facts and hypotheses for multi-professional considerations in predictive, preventive and personalised medicine. EPMA Journal. 8(1): 23-33.
- Castellanos G, Bernabé-García Á, Moraleda JM, Nicolás FJ (2017) Amniotic membrane application for the healing of chronic wounds and ulcers. Placenta. 59: 146-153.
- Ramuta TŽ, Tratnjek L, Janev A, Seme K, Erjavec MS, et al. (2021) The antibacterial activity of human amniotic membrane against multidrug-resistant bacteria associated with urinary tract infections: New insights from normal and cancerous urothelial models. Biomedicines. 9(2): 218.
- McQuilling JP, Vines JB, Kimmerling KA, Mowry KC (2017) Proteomic Comparison of Amnion and Chorion and Evaluation of the Effects of Processing on Placental Membranes. Wounds. 29(6): E36-E40.
- Avilla-Royo E, Gegenschatz-Schmid K, Grossmann J, Kockmann T, Zimmermann R, et al. (2021) Comprehensive quantitative characterization of the human term amnion proteome. Matrix Biol Plus. 12: 100084.
- Grzywocz Z, Pius-Sadowska E, Klos P, Gryzik M, Wasilewska D, Aleksandrowicz B, et al. (2014) Growth factors and their receptors derived from human amniotic cells in vitro. Folia Histochem Cytobiol. 52(3): 163-170.