Mirkhalig Javadov MD1*, Aygun Gojayeva MD2, Zeynab Allahverdiyeva MD3, Elnura Gasimova MD3, Nahida Gurbanova MD4, Elshan Gadimov MD1, Aykhan Abbasov MD1
1Yeni Klinika Hospital, Department of General Surgery, Tabib Academy, Baku, Azerbaijan. ORCID: 0000-0002-4288-0400
2Yeni Klinika Hospital, Tabib Academy, Baku, Azerbaijan
3Central Clinic Hospital, Department of Oncology and Hematology, Baku, Azerbaijan
4Central Clinic Hospital, Department of Radiology, Baku, Azerbaijan
*Corresponding Author: Mirkhalig Javadov MD, Yeni Klinika Hospital, Department of General Surgery, Tabib Academy, Baku, Azerbaijan. ORCID: 0000-0002-4288-0400
Abstract
Background: Small intestinal adenocarcinoma is a rare gastrointestinal malignancy with non-specific symptoms and typically late diagnosis. Peritoneal carcinomatosis indicates advanced-stage disease and is associated with poor prognosis. Palliative chemotherapy is the standard treatment for patients with metastatic small intestinal adenocarcinoma. This case report highlights the treatment of a 38-year-old female with peritoneal carcinomatosis secondary to metastatic adenocarcinoma with signet ring cell features originating from the lower gastrointestinal tract. Small intestinal signet ring cell adenocarcinoma is a rare, aggressive form of cancer that originates particularly from the ileum.
Case Presentation: We present a 38-year-old female patient with a one-year history of fatigue, anemia, intermittent abdominal pain and nausea. The patient underwent emergency laparotomy with the diagnosis of acute abdomen and intestinal obstruction. Small bowel resection was done with the diagnosis of adenocarcinoma (T4N1bM1), p53(-), KRAS (-), BRAF (-), PD-L1 30 % mutations with peritoneal dissemination. She was hospitalized for further evaluation and received FOLFOX and Altuzan-based chemotherapy. Despite initial treatment, disease progression led to diffuse peritoneal metastases and ascites. Recently, the systemic screening was performed on the patient and liver, lung, bone and brain metastases were detected.
Conclusion: This case report highlights the diagnostic and therapeutic challenges of small intestinal adenocarcinoma and underscores the importance of early detection and a multidisciplinary approach.
Keywords: Small intestine adenocarcinoma, peritoneal carcinomatosis, ascites, chemotherapy, signet ring cell
Introduction
Small intestinal adenocarcinoma (SIA) is a rare malignancy, accounting for less than 5% of all gastrointestinal cancers [1]. Due to the non-specific and insidious nature of its symptoms--such as fatigue, abdominal pain, and weight loss-it is often diagnosed at advanced stages, frequently with distant metastases or peritoneal carcinomatosis. Unlike colorectal or gastric cancers, there is limited data guiding optimal treatment strategies for SIA, and survival rates remain poor, particularly in metastatic cases.
Signet-ring cell carcinoma (SRCC) is a rare subtype of adenocarcinoma that is identified most frequently in the stomach, where it accounts for 25% of all gastric cancers [2]. SRCC has also been detected in the pancreas, breast, bladder, ovaries, esophagus, lungs and large intestine. The five-year survival rate of patients diagnosed with SRCC has been estimated at 20–30%.
Small bowel adenocarcinoma remains one of the most prevalent malignancies worldwide and is often diagnosed at advanced stages, particularly when initial symptoms are nonspecific. In some cases, patients present with acute abdominal conditions such as intestinal obstruction, which may reveal underlying malignancy. Despite standard treatment protocols involving chemotherapy regimens like FOLFOX and targeted agents such as Bevacizumab, disease progression and metastasis remain significant challenges, especially in aggressive or mucinous subtypes.
We present a case of a patient initially operated for intestinal obstruction, later found to have small bowel cancer with rapid progression, peritoneal carcinomatosis, and widespread metastases involving the lungs, liver, bones, and brain. The case highlights the aggressive nature of certain small bowel tumors and the limitations of conventional treatment approaches.
Peritoneal carcinomatosis (PC) represents a challenging manifestation of disease spread, associated with ascites, reduced quality of life, and limited therapeutic options. Recent advances in chemotherapy and targeted therapies offer some promise, but the prognosis remains guarded.
In this report, we present a rare case of SIA with peritoneal carcinomatosis in a young female patient, highlighting the clinical course, diagnostic challenges, and response to FOLFOX-based chemotherapy. This case contributes to the growing but still limited literature on effective management approaches in advanced SIA.
Case Presentation
A 38-year-old female with no significant family history of cancer was admitted to the oncology department with complaints of persistent fatigue, pallor, dizziness and colicky abdominal pain. Her minor symptoms had started approximately one year prior to admission and had progressively worsened.
In July 2022, she underwent surgical resection of the small intestine due to obstructive symptoms and was diagnosed histopathologically with adenocarcinoma (Figure 1). Small bowel resection was done with the diagnosis of adenocarcinoma (T4N1bM1), p53(-), KRAS (-), BRAF (-), PD-L1 30 % mutations with peritoneal dissemination. Adjuvant chemotherapy was prescribed to the patient, but she did not accept oncological treatment. A year later, the patient presented again with severe abdominal pain and a hemoglobin level of 6 g/dl. She received two cycles of treatment with FOLFOX and the patient voluntarily discontinued oncological treatment. However, she returned for the third cycle of treatment with complaints of abdominal pain and signs of peritoneal carcinomatosis. The previously observed 3–4 cm tumor had grown to approximately 22 cm. In addition, a mass was identified in the ovarian fossa, along with mucinous lesions.
After a further 7–8 months, the patient returned back for treatment again and was prescribed three cycles of FOLFOX in combination with Bevacizumab. Despite this, disease progression was continued, and metastases were detected in the lungs, liver, bones, and brain. Additionally, the patient presented with peritoneal ascites, arrhythmia, and panic attacks, and was under observation for status epilepticus.
Small bowel obstruction is a significant surgical emergency frequently encountered in daily clinical practice. In approximately 60% of cases, the most common underlying cause is postoperative adhesions. Although less common, strictures secondary to small bowel adenocarcinoma can also lead to small bowel obstruction.
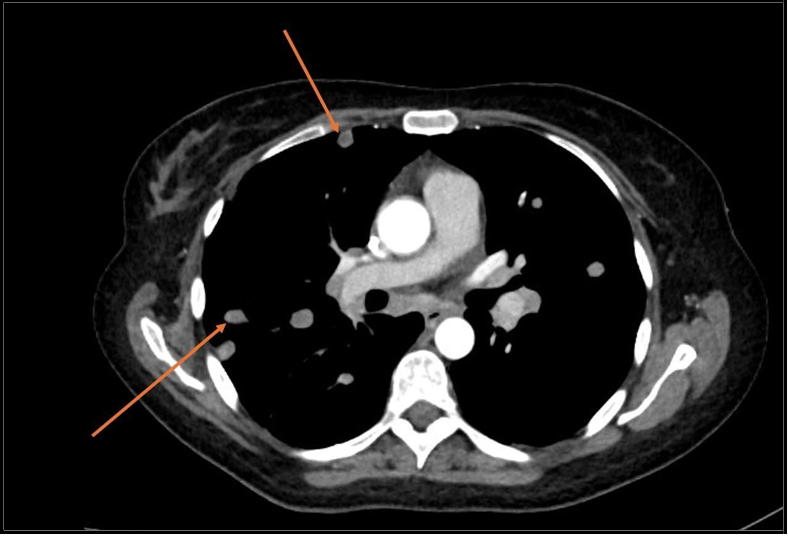
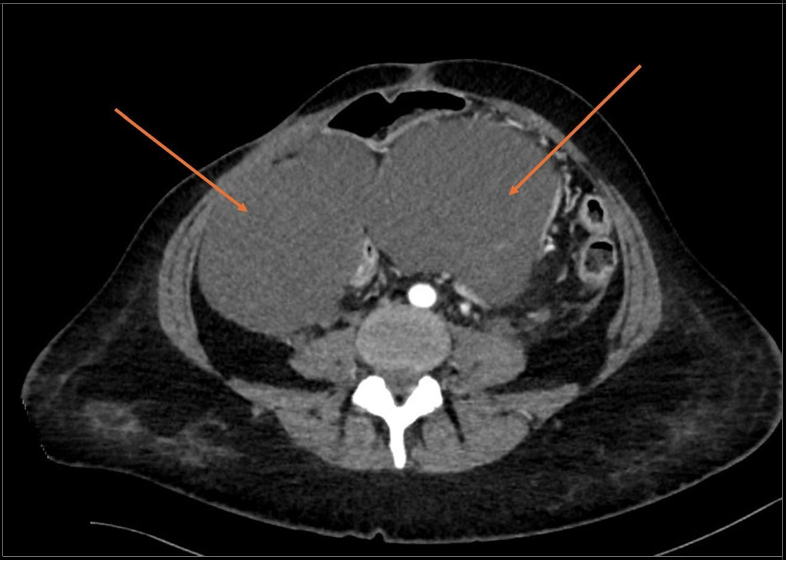
CT scan showed that metastases were present in distant organs (Figure 2, 3).
She was hospitalized for further evaluation and received FOLFOX and Altuzan-based chemotherapy.
Recently, the systemic screening was performed on the patient and liver, lung, bone and brain metastases were detected.
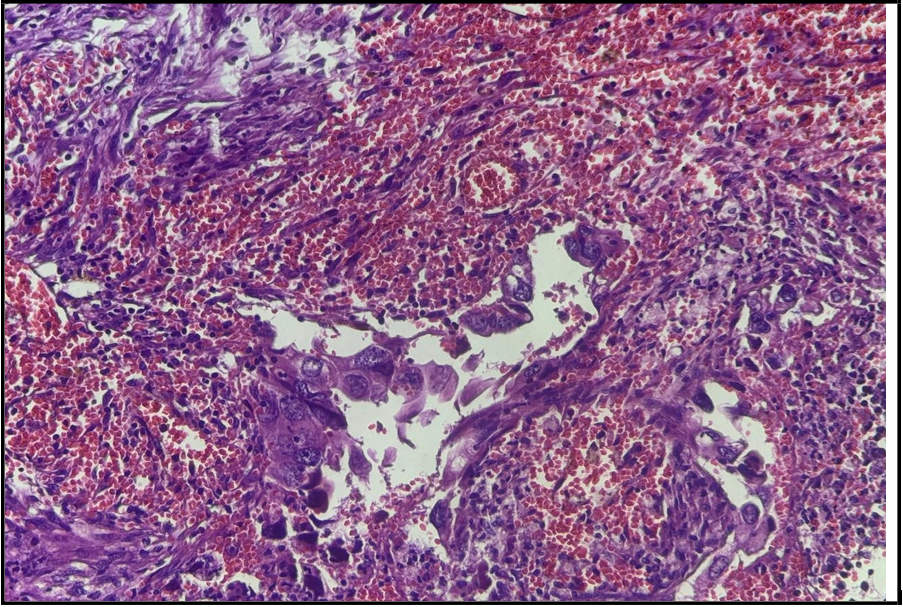
Figure 1: Representative photos of the tumor specimen with hematoxylin and eosin staining, revealing moderately to poorly differentiated adenocarcinoma
Figure 2: Chest CT scan Lung metastasis shown with arrows
Figure 3: Abdominal CT scan arrows shows abdimonal ascites.
Discussion
Small intestinal adenocarcinoma (SIA) is a rare and under-recognized malignancy, often diagnosed late due to vague and non-specific symptoms [1]. Unlike colorectal or gastric cancers, there are no widely accepted screening strategies or treatment guidelines specifically tailored for SIA. Signet-ring cell carcinoma (SRCC) is a rare subtype of adenocarcinoma that is identified most frequently in the stomach, where it accounts for 25% of all gastric cancers [2].
Consequently, many patients, as in this case, present with advanced disease and peritoneal spread at the time of diagnosis. Peritoneal carcinomatosis (PC) in the setting of SIA is associated with poor prognosis [3], with median overall survival typically less than one year. Palliative chemotherapy was administered to 38% of patients with metastatic disease [4]. Treatment options are largely extrapolated from colorectal cancer protocols, including FOLFOX or FOLFIRI regimens, with or without targeted therapies such as Bevacizumab [5]. Our patient initially underwent surgery due to intestinal obstruction. Histopathological analysis of the pathological result confirmed the presence of an oncological disease. Adjuvant chemotherapy was prescribed to the patient, but she did not accept oncological treatment. A year later, the patient presented again with severe abdominal pain and a hemoglobin level of 6 g/dl. She received two cycles of treatment with FOLFOX and the patient voluntarily discontinued oncological treatment. However, she returned for the third cycle of treatment with complaints of abdominal pain and signs of peritoneal carcinomatosis. The previously observed 3–4 cm tumor had grown to approximately 22 cm. In addition, a mass was identified in the ovarian fossa, along with mucinous lesions.
After a further 7–8 months, the patient returned back for treatment again and was prescribed three cycles of FOLFOX in combination with Bevacizumab. Despite this, disease progression was continued, and metastases were detected in the lungs, liver, bones, and brain. Additionally, the patient presented with peritoneal ascites, arrhythmia, and panic attacks, and was under observation for status epilepticus.
Small bowel obstruction is a significant surgical emergency frequently encountered in daily clinical practice. In approximately 60% of cases, the most common underlying cause is postoperative adhesions. Although less common, strictures secondary to small bowel adenocarcinoma can also lead to small bowel obstruction [6]. Although the prevalence of adenomas in the small intestine is lower than in the colon, a similar phenotypic adenoma–carcinoma sequence appears to exist. Notably, a key distinction lies in the infrequent occurrence of mutations in the adenomatous polyposis coli (APC) gene. Evidence suggests that small bowel adenocarcinoma may follow a distinct genetic pathway compared to colorectal cancer. In one study analyzing 21 nonfamilial, nonampullary small bowel adenocarcinomas, no mutations were detected in the mutation cluster region of the APC gene. However, abnormal expression of E-cadherin and beta-catenin was observed, suggesting a potential alternative early molecular event in the tumorigenesis of small bowel adenocarcinoma, independent of APC gene alterations [7].
The case also demonstrates the severity of anemia in advanced gastrointestinal malignancies. This highlights the importance of regular hematologic monitoring and early nutritional and supportive interventions. Nevertheless, more than 30% of treated patients develop a disease recurrence, with a disease-free survival of approximately 2 years [8].
Symptomatic metastases in the small intestine often necessitate surgical intervention typically resection of the affected segment, and occasionally the creation of a primary enterostomy. Unfortunately, the overall prognosis remains very poor. Despite advances in medical and surgical approaches, treatment for peritoneal carcinomatosis is still largely palliative, with median survival rarely exceeding two months. Published literature reports perioperative mortality rates ranging from 60% to 100% [9].
In small bowel adenocarcinoma (SBA), symptoms typically include abdominal discomfort, anemia due to occult or overt bleeding, weight loss, nausea, vomiting, and occasionally obstructive pain. In our case, the patient presented with abdominal pain and anemia compounded by vomiting and weight loss, mirroring common SBA presentations. Diagnostic imaging with conventional CT, while insufficient for definitive differential diagnosis, plays a critical role in staging by distinguishing benign from malignant lesions. In this instance, CT revealed an irregular circumferential mass with shouldered margins and upstream dilatation classic radiological signs of malignancy [10] Capsule endoscopy (CE) excels at detecting scattered, small, or multiple lesions and active bleeding in the small intestine. It is minimally invasive, well-tolerated, and comfortable for patients. However, as a purely diagnostic tool, it lacks therapeutic capability, has no biopsy function, and may miss lesions due to rapid transit or poor bowel preparation.
In contrast, double-balloon enteroscopy (DBE) enables directed visualization, biopsy, and therapeutic intervention (e.g., hemostasis, dilation). Yet it is less patient-friendly: requiring sedation or general anesthesia, longer procedure time, and sometimes incomplete small- bowel traversal. DBE’s invasiveness reduces tolerance and completion rates, impacting diagnostic yield.
A combined diagnostic strategy is therefore recommended: initial evaluation with CE owing to its non-invasive nature and high diagnostic yield followed, if warranted, by DBE to confirm findings histopathologically or perform interventions.
Baseline levels of carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9) are important prognostic markers, particularly in advanced disease. In our case, a multimodal diagnostic approach was employed: findings from CT and PET/CT imaging, and elevated CEA and CA 19-9 values were integrated to establish the diagnosis. Despite limited treatment options, selected patients may benefit from systemic chemotherapy, targeted agents, and a multidisciplinary approach involving surgery, oncology and palliative care. In our case, initiation of FOLFOX plus Altuzan led to temporary stabilization of the patient's condition and allowed for discharge with outpatient follow-up planning [11, 12].
This case emphasizes the need for greater awareness of small bowel cancers, consideration of peritoneal involvement in symptomatic patients, and more structured therapeutic algorithms in this patient population.
Further research and case documentation are crucial to better define prognostic indicators and improve outcomes in SIA.
Conclusion
Small Intestinal adenocarcinoma with peritoneal carcinomatosis remains a fare and aggressive malignancy with limited treatment options and poor prognosis. Early recognition, timely surgical intervention, and appropriate systemic therapy may offer temporary disease control. This case highlights the importance of individualized treatment planning and the need for further clinical research to establish standardized protocols for such patients.
References
- Linssen JDG, Schafrat PJM, de Back TR, van Erning FN, van Leerdam ME, et al. (2025) Predisposing conditions in patients with small intestinal adenocarcinomas in the Netherlands: A 20- year nationwide cohort study. Int J Cancer. 157(2): 218-231.
- Martins T, Umar J, Groudan K, Bharadwaj HS, Desilets D (2022) Mucinous Signet-Cell Adenocarcinoma of the Ileum: A Diagnostic Challenge-Case Report and Review of the Literature. Case Rep Gastrointest Med. 2022: 5703407.
- Singh RSS, Hisham MRM, Mohammad AT, Fazle Abbas SH, Yusoff Y (2020) An unusual case of primary peritoneal adenocarcinoma. Int Surg J. 7(10): 3445–3448.
- Legué LM, Bernards N, Lemmens VE, de Hingh IH, Creemers GJ, et al. (2019) Palliative chemotherapy for patients with synchronous metastases of small-bowel adenocarcinoma: A reflection of daily practice. United European Gastroenterol J. 7(10): 1380-1388.
- Obayashi M, Otsuka S, Ashida R, Ohgi K, Yamada M, et al. (2023) Conversion surgery for advanced jejunal adenocarcinoma with multiple peritoneal metastases: a case report. Surg Case Rep. 9(1): 145.
- Bouali M, Sylvestre K, Benghait H, El Bakouri A, El Hattabi K, et al. (2022) Small bowel adenocarcinoma a rare cause of upper gastrointestinal obstruction (a case report and literature review). Int J Surg Case Rep. 91: 106763.
- Umman P, Adiyodi V, Narayan C (2013) Small bowel adenocarcinoma-report of two cases and review of literature. Indian J Surg. 75(2): 123-127.
- Farina M, Falbo F, Biancucci A, Lucandri G, Pende V, et al. (2022) Small bowel adenocarcinoma: natural history of recurrence after surgical resection. J Surg Case Rep. 2022(10): 451.
- Tan T, Huang Y, Yan R, Liu Y (2025) Case Report: Peritoneal and small bowel metastasis from lung adenocarcinoma with EGFR L858R mutation. Front Oncol. 15: 1423520.
- Khosla D, Dey T, Madan R, Gupta R, Goyal S, et al. (2022) Small bowel adenocarcinoma: An overview. World J Gastrointest Oncol. 14(2): 413-422.
- Alherz F, Al Omoush TM, Alenezi NH, Albalawi TF, Alsaif O (2022) Primary Adenocarcinoma of the Jejunum: Case Report of Rare Small Bowel Neoplasm. Cureus. 14(12): e33032.
- Li J, Wang Z, Liu N, Hao J, Xu X (2016) Small bowel adenocarcinoma of the jejunum: a case report and literature review. World J Surg Oncol. 14(1): 177.