Kasid A. Nouri. MD1, Mallika Mehrotra2*, Jeyaseelan Lakshmanan PhD2
1From Department of Neurology, Mediclinic Welcare Hospital, Dubai, UAE
2From Mohamed Bin Rashid University of Medicine and Health Science, Dubai, UAE
*Corresponding Author: Mallika Mehrotra, From Mohamed Bin Rashid University of Medicine and Health Science, Dubai, UAE.
Abstract
Introduction
SARS-CoV-2 has caused a worldwide pandemic due to its high transmission rate among humans and causing a threat to global health. Numerous recent research suggests that SARS-CoV-2 has detrimental effects on the brain processes and may even cause significant neurological impairment during this continuing contagion. Some of the main neuro-invasion pathways through which SARS-CoV-2 can cause neurologic events are direct invasion, hematogenic routes, hypoxia, and cytokine storm.
Objectives
We detailed the potential pathways for SARS-CoV-2 entry into the nervous system, the subtypes of neurological manifestations, the underlying comorbidities, and the critical variables that induced them. The central nervous system (CNS) effects of the SARS-CoV-2 infection are evaluated in their neurological manifestations.
Methods
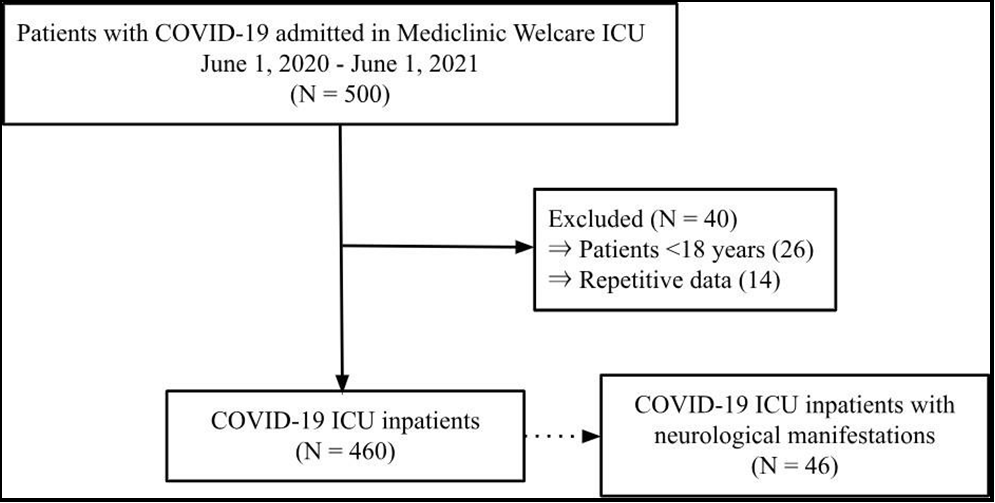
In the current work, we collected the data of 500 COVID-19 inpatients from the HIS database from June 2020 to June 2021. From this, 460 inpatients met the inclusion criteria, and amongst them, it was found that 46 COVID-19 patients developed neurological manifestations.
Results and Conclusion
Through the data collected, we analyzed that the majority of the patients who were afflicted by such neurologic events had a mean age of 63 years ± 16 years and had associated comorbidities such as diabetes, hypertension, and cardiovascular diseases. The most common neurologic subtype was ischemic stroke, with diabetes being the most prevalent comorbidity.
Introduction
The World Health Organization (WHO) proclaimed on May 5th, 2023, that SARS-CoV-2 is an ongoing health issue that no longer constitutes a public health emergency. However, since the commencement of the epidemic, the WHO has reported 6.5 million COVID-19 deaths and over 633 million cumulative cases globally.
The hematogenous and neuronal retrograde routes are the two major pathways through which SARS-CoV-2 can enter the central nervous system (CNS). SARS-CoV-2 increases vascular permeability through multiple mechanisms. When the virus enters the cells, it initially binds to the human angiotensin-converting-enzyme receptor 2 (ACE2) on the cell surface. This reduces the expression and activity of ACE2 and indirectly activates the kallikrein-bradykinin pathway (KKS) [28]. KKS plays an essential role in endothelial pathogenesis. In normal human physiology, KKS exterminates pathogens by recruiting neutrophils, and spare bradykinin is degraded by ACE, but when there is a decrease in ACE2 activity, it leads to an excess of bradykinin, which causes an increase of activated neutrophils [6]. Neutrophils produce reactive oxygen species (ROS), damaging tissue and causing vascular leakage. When SARS-CoV-2 infects the endothelial cells of the blood-brain barrier (BBB), it leads to vascular leakages through the pathogenesis mentioned above and, therefore, permits the virus to enter the blood-CSF barrier. SARS-CoV-2 penetrates the CNS via the “Trojan Horse” mechanism, where the virus infects leukocytes, mainly monocytes, as they express ACE2 receptors, and the infected immune cell can migrate through the paracellular route across the permeable BBB and into the cerebrospinal fluid (CSF) [4]. However, according to (Frontera et al., 2021), there is no evidence of SARS-CoV-2 in the CSF of RT-PCR-positive patients. SARS-CoV-2 can also invade the CNS through a neural retrograde route, where the virus travels through retrograde axonal transport to reach the neuron cell bodies of the central nervous system [15]. Recent research indicates headaches, mental confusion, strokes, and rarely encephalitis may be early signs and symptoms that suggest neurotropism in SARS-CoV-2 [42].
This study aims to establish the frequency of COVID-19 ICU inpatients developing neurological manifestations, which is the outcome of interest, and what underlying exposures, such as associated comorbidities, can predispose the inpatients to build this.
Methodology
Research design
A retrospective cohort study was conducted, which included COVID-19 ICU inpatients in Mediclinic Welcare Hospital. Dubai, UAE, between June 1st, 2020, to June 1st, 2021. Mediclinic Welcare was proclaimed a COVID-19 ICU, making it the choice for data collection.
Participants
The inclusion criteria for this study were inpatients who tested reverse transcriptase PCR (RT-PCR)- positive for SARS-CoV-2 infection, as it was the most sensitive and specific test for detecting COVID-19 and it reduced the risks of a false positive [14]. Furthermore, this study focused on COVID-19 patients admitted into the hostile pressure intensive care unit (ICU), as most of the severe COVID-19 inpatients were welcomed there, allowing us to collect the data on inpatients with a higher chance of developing neurological manifestations. Moreover, we only included COVID-19 inpatients with neurological manifestations who were assessed based on the neurological assessment chart [2] done by a consultant neurologist and had neuroimaging, EEG, or neurophysiological studies done.
The exclusion criteria for this study were inpatients aged≥ 18 years. The majority of COVID-19 inpatients ≤18 years had a different disease presentation compared to inpatients ≥18 years [11].
Moreover, inpatients ≤18 years would have other exposures than the underlying comorbidities this research aims to find. Additionally, repetitive data was also excluded from the data collection. The records of 500 COVID-19 inpatients were collected, and after acknowledging the inclusion and exclusion criteria, we had data of 460 COVID-19 ICU inpatients. Among the 460 inpatients, 46 developed neurological manifestations during their hospitalization.
Equipment and materials
The equipment used for this research were secure desktop computers found in Mediclinic Welcare Hospital. The data was collected using the HIS database. The database comprises information on the patient’s demographics, such as gender, age, and nationality. It also collected data on their past medical records, underlying chronic comorbidities, allergies, and vital status. Furthermore, it contained the data of the patient’s vitals, complete blood count (CBC), and chest scans to prove COVID-19 pneumonia. The data found on the database was already recorded, making it scored data. This data was then analyzed on Statistical Package for The Social Sciences (SPSS) to find the significance between the development of neurological events and the potential exposures that could have led to the outcome of interest.
Results
In this retrospective cohort study, of the 460 COVID-19 ICU inpatients (62.0% men), 46 of these inpatients presented with neurological symptoms.
Figure 1: Flow chart of study enrolment
The most common subtypes of primary neurologic events seen were Ischemic stroke (60.9%), polyneuropathy (19.6%), seizures (10.9%), and haemorrhagic stroke (6.5%).
Table 1: Mean and Standard deviation for continuous data
|
|
Neurological manifestations |
|
|||||||||
|
Yes |
No |
|
|||||||||
|
Mean |
SD |
Median |
Percentile |
Mean |
SD |
Median |
Percentile |
P Value |
|||
|
|
|
|
25 |
75 |
|
|
25 |
75 |
|
||
|
Age |
63 |
16 |
64 |
56 |
74 |
50 |
14 |
49 |
40 |
59 |
0.000 |
|
Hospital duration |
71 |
67 |
48 |
32 |
91 |
14 |
31 |
7 |
4 |
11 |
0.000 |
|
BMI |
31 |
9 |
28 |
26 |
32 |
30 |
6 |
29 |
26 |
32 |
0.443 |
|
WBC |
12.0 |
6.1 |
13.4 |
6.7 |
16.1 |
7.4 |
4.4 |
6.2 |
4.7 |
8.8 |
0.000 |
The mean hospital duration for COVID-19 patients without neurological symptoms was 14 days ± 31, with a median of 7 days. However, the mean for inpatients who developed neurological events was 71 ± 67, with a median of 48 days. There is a significant difference between the hospital duration for COVID-19 in patients who developed neurological events compared to inpatients who did not, and this can be supported by a P<0.05 showing the significance. Even though there is a significant difference between the means, the standard deviations are enormous for both groups, indicating a substantial variance in the data, signifying that the data is spread out. Therefore, affecting the reliability of the data collected.
Furthermore, there is a significant difference between the mean ages of COVID-19 inpatients who did and did not develop neurological consequences. Patients who did not develop neurological manifestations had a mean age of 50 years ± 14, whereas patients who did develop neurological manifestations had a mean age of 63 years± 16. This shows that, on average, patients with neurological symptoms were older than patients without. This can be supported by the (p<0.05), signifying the link between these two variables.
The BMI of COVID-19 inpatients with neurological manifestations is 31 ± 9 compared to inpatients without any manifestations being 30 ± 6. There isn’t a significant difference between the BMIs, and it can also be seen that the medians are very similar, with 28 and 29 BMI, respectively. This is further proven by (P>0.05), showing that the variable is insignificant.
It can also be seen that patients who developed neurological symptoms had a greater mean white blood cell count (WBC) of 12 ± 6.1 as compared to inpatients who did not, with a mean WBC of 7.4± 4.4. According to WHO, the range of WBC should be between 4.5 to 11.0 × 109/L, and the mean and median WBC of COVID-19 patients who developed neurological symptoms surpasses that with a WBC of 12 and 13.4, respectively. The (p<0.05) shows a significant correlation between the two variables. A higher WBC count correlates with a more severe infection, thus signifying that patient with neurological manifestations had a more severe COVID-19 illness. Moreover, the standard deviation is slight for both groups; therefore, it is clustered around the mean and thus increases the reliability of the data.
Table 2: Status of COVID-19 inpatients with and without neurological manifestations
|
Status of COVID-19 inpatients |
|
|||||
|
|
Status |
Total |
P value |
|||
|
Alive |
Dead |
|||||
|
Neurological Manifestations |
Yes |
Count |
32 |
14 |
46 |
0.000 |
|
% |
69.6% |
30.4% |
100.0% |
|||
|
No |
Count |
397 |
17 |
414 |
||
|
% |
95.9% |
4.1% |
100.0% |
|||
|
Total |
Count |
429 |
31 |
460 |
||
|
% |
93.3% |
6.7% |
100.0% |
|||
The table above shows that the total mortality of COVID-19 ICU inpatients is 6.7% (95% CI: 4.4, 9.0). However, the mortality rate of COVID-19 ICU inpatients who developed neurological events was 30.4%, whereas patients who did not create such events had a mortality of 4.1%. This shows that there was a significant increase in mortality rate when a patient had a neurological manifestation, and this can be further supported by a (P<0.05). Furthermore, in (Frontera et al., 2021), it can be seen that COVID-19 in patients who developed neurological disorders had a mortality of 35%, which is close to the mortality rate seen in Mediclinic Welcare Hospital.
However, in (Frontera et al., 2021), the mortality rate for COVID-19 inpatients without any neurological disorders is 19%, whereas in Mediclinic Welcare Hospital, it is 4.1%. There is no definitive reason for such a remarkable difference in the mortality rates. However, this difference could be because of the sample size. In (Frontera et al., 2021), the sample size is 4,491, whereas in this paper, it is 460 inpatients.
According to Table 3, there is no significance between the gender of the patient and whether they will develop neurological consequences. This might be because, in both groups, there is a male predominance, causing asymmetrical distribution and leading to skewed data.
Looking at (Table 3). it can be noted that the most significant comorbidities that could have predisposed COVID-19 inpatients to develop neurological symptoms are diabetes, cardiovascular diseases, and hypertension. Renal disease and hematological conditions. This is proven by (P<0.05).
Table 3: Association Between Co-morbidities and Neurological Manifestations
|
|
Neurological Manifestations |
|
||||
|
Yes |
No |
P value |
||||
|
N |
% |
n |
% |
|||
|
Gender |
Male |
25 |
8.8% |
260 |
91.2% |
0.168 |
|
Female |
21 |
12.0% |
154 |
88.0% |
|
|
|
Diabetes |
Yes |
34 |
23.3% |
112 |
76.7% |
0.000 |
|
No |
12 |
3.9% |
299 |
96.1% |
|
|
|
Cardiovascular disease |
Yes |
20 |
17.9% |
92 |
82.1% |
0.002 |
|
No |
26 |
7.5% |
319 |
92.5% |
|
|
|
Hypertension |
Yes |
32 |
19.3% |
134 |
80.7% |
0.000 |
|
No |
14 |
4.8% |
277 |
95.2% |
|
|
|
Renal disease |
Yes |
7 |
30.4% |
16 |
69.6% |
0.005 |
|
No |
39 |
9.0% |
395 |
91.0% |
|
|
|
Gastro-hepaticconditions |
Yes |
4 |
17.4% |
19 |
82.6% |
0.192 |
|
No |
42 |
9.7% |
392 |
90.3% |
|
|
|
Haematologicalconditions |
Yes |
6 |
35.3% |
11 |
64.7% |
0.004 |
|
No |
40 |
9.1% |
400 |
90.9% |
|
|
|
Autoimmuneconditions |
Yes |
3 |
21.4% |
11 |
78.6% |
0.158 |
|
No |
43 |
9.7% |
400 |
90.3% |
|
|
|
Past surgery |
Yes |
5 |
12.8% |
34 |
87.2% |
0.354 |
|
No |
41 |
9.8% |
377 |
90.2% |
|
|
|
Secondary infections |
Yes |
29 |
46.8% |
33 |
53.2% |
0.000 |
|
No |
16 |
4.0% |
380 |
96.0% |
|
|
Acute stroke
Acute stroke is classified into 2 subcategories, either ischemic or hemorrhagic stroke. As seen in Table 10. the most common neurological event in COVID-19 inpatients was stroke. Around 60.9% of COVID-19 inpatients with neurological events present with ischemic stroke as their primary manifestation (Table 10). and 6.5% current with hemorrhagic stroke Table 11.
The patients were clinically diagnosed with acute stroke using either computed tomography (CT_, magnetic resonance imaging (MRI), or angiogram. It also helped determine whether the patient was suffering from an ischemic or hemorrhagic attack. Looking at Table 6. we can see that patients who had a CT scan done have a lower mortality. This is also proven by (P<0.05), making the variable significant and concluding that a CT scan should improve survival rates.
Table 6 shows that an MRI or cerebral angiogram does not significantly affect the mortality rate (P>0.05). According to the article (Vymazal et al., 2012), patients with an acute stroke should be first evaluated by a CT scan as an MRI is more complicated and time-consuming, which that patient might not at that moment [32]. We are not considering angiogram as that was not the primary modality of diagnosis for patients with acute stroke. There is no significant correlation between the angiogram and the patient's status. While looking into the most efficient modality for diagnostic purposes, it is crucial to note which medication should be given to inpatients with stroke to improve their mortality rate. According to Table 6. the most valuable drugs to improve the mortality of COVID-19 inpatients with neurological manifestations are aspirin and enoxaparin, a low molecular weight heparin (LMWH). One of the main reasons why enoxaparin has a higher survival rate in comparison to heparin is because of its bioavailability, which is around 90% when given subcutaneously [36]. On the other hand, aspirin is a nonsteroidal anti-inflammatory drug which is also antiplatelet. As seen in (Table 6). aspirin also decreases the mortality rate of patients with stroke. Aspirin helps prevent early recurrent ischemic stroke (Coull et al. 2002) [37]. Therefore, it was always administered with enoxaparin or heparin; according to the data, it was only administered without any adjunct, and the patient did not survive, although the sample size is minimal to conclude this statement. However, aspirin does improve survival rate (P<0.05). Lastly, it can be noted from (Table 7). that there is no significant correlation between the past medical history of stroke and an acute stroke incident in COVID-19 inpatients.
Table 4: Frequency of seizures in COVID-19 inpatients
|
|
Seizures |
|
|
|
Yes |
No |
|
Epilepsy relapse |
2 4.3% |
44 95.7% |
|
Generalized Seizures |
11 23.9% |
35 76.1% |
Table 5: Types of seizures according to EEG
|
Type of seizure |
|||||
|
|
Frequency |
Percent |
Valid Percent |
CumulativePercent |
|
|
Valid |
|
1 |
2.2 |
2.2 |
2.2 |
|
Generalized epilepsy |
2 |
4.3 |
4.3 |
6.5 |
|
|
Generalized seizures (generalized slowing) |
8 |
17.4 |
17.4 |
23.9 |
|
|
No |
34 |
73.9 |
73.9 |
97.8 |
|
|
PRES syndrome |
1 |
2.2 |
2.2 |
100.0 |
|
|
Total |
46 |
100.0 |
100.0 |
|
|
Table 6: Management and modality of diagnosis inpatients with stroke and seizure with regards tostatus
|
|
Alive |
Dead |
P value |
|
|
Levetiracetam_seizure |
No |
1 |
0 |
0.000 |
|
Yes |
6 |
2 |
||
|
EEG_seizure |
No |
1 |
1 |
0.001 |
|
Yes |
6 |
1 |
||
|
CT Scan_Stroke |
No |
4 |
2 |
0.002 |
|
Yes |
16 |
9 |
||
|
MRI Scan_stroke |
No |
7 |
5 |
0.076 |
|
Yes |
13 |
6 |
||
|
Cerebral angiogram_stroke |
No |
18 |
11 |
0.473 |
|
Yes |
2 |
0 |
||
|
Clexane_Stroke |
No |
2 |
5 |
0.045 |
|
Yes |
18 |
6 |
||
|
Aspirin_Stroke |
No |
8 |
5 |
0.001 |
|
Yes |
12 |
6 |
||
|
Heparin_Stroke |
No |
20 |
9 |
0.169 |
|
Yes |
0 |
2 |
||
Table 7: Past history of stroke and acute stroke
|
|
Ischemic Stroke |
P value |
|||||
|
No |
Yes |
||||||
|
Past stroke |
No |
14 |
24 |
0.972 |
|||
|
Yes |
3 |
5 |
|||||
|
Haemorrhagic stroke |
|||||||
|
Past stroke |
No |
35 |
3 |
0.674 |
|||
|
Yes |
7 |
0 |
|||||
Table 8: Nationality of COVID-19 inpatients
|
|
Neurological Manifestations |
|
||||
|
Yes |
No |
P value |
||||
|
Count |
Row N % |
Count |
Row N % |
|||
|
Nationality |
Middle East |
18 |
13.6% |
114 |
86.4% |
0.024 |
|
South Asia |
27 |
10.4% |
233 |
89.6% |
|
|
|
Others |
1 |
1.5% |
67 |
98.5% |
|
|
Table 9: D-dimer levels of COVID-19 inpatients with neurological manifestations
|
|
StatusR |
N |
Mean |
Std. Deviation |
P Values |
|
D Dimer on incident |
Alive |
30 |
4107.20 |
4964.359 |
0.047 |
|
Dead |
13 |
7372.69 |
6052.277 |
|
Table 10: Primary neurological manifestations
|
|
Frequency |
Percent |
Valid Percent |
Cumulative Percent |
|
|
Valid |
Encephalopathy |
1 |
2.2 |
2.2 |
2.2 |
|
Haemorrhagicstroke |
3 |
6.5 |
6.5 |
8.7 |
|
|
Ischemic stroke |
28 |
60.9 |
60.9 |
69.6 |
|
|
Polyneuropathy |
9 |
19.6 |
19.6 |
89.1 |
|
|
Seizure |
5 |
10.9 |
10.9 |
100.0 |
|
|
Total |
46 |
100.0 |
100.0 |
|
|
Seizures
We are looking at Table 10. we can see that around 10.9% of COVID-19 inpatients with neurological manifestations present with seizures as the main neurological incident. Moreover, 8.7% present with seizures as their second neurological manifestation (Table 11).
Seizures were diagnosed clinically using an electroencephalogram (EEG) or neuroimaging. Through this, we were able to subcategorize the seizures into epilepsy or generalized seizures. Looking at (Table 4). we can determine the frequency of each subcategory. 11 COVID- 19 inpatients developed generalized seizures, of which 2 further developed epilepsy.
Amongst these 2 inpatients, both had a past medical history of epilepsy. One of them developed generalized epilepsy, whereas the other patient developed posterior reversible encephalopathy syndrome (PRES syndrome). Some literature suggests that SARS-CoV-2 can lead patients to have an inflammatory and hypercoagulable state, which could result in them developing PRES syndrome [43].
We are, furthermore, looking at table 5. we can see that the most common type of seizure is generalized seizure, where the EEG suggests generalized slowing activity. However, there is no definitive diagnosis for this generalized slowing after these patients presented with a seizure.
For the management of these seizures, the patients were given levetiracetam, which is an antiepileptic drug [35]. According to Table 6. patients have a higher survival rate when administered with levetiracetam, and this is proven by (P<0.05). Furthermore, Table 6. also explores the survival rate of inpatients with seizures who had an EEG done as a diagnostic. Amongst the two inpatients who didn't have an EEG done, one of them passed away, making the mortality rate 50%. Although the numbers are minimal, this is not a conclusive statement. However, the inpatients who did get an EEG had a mortality of 16.7%, and this can be supported by a (P<0.05).
Table 11: Secondary neurological manifestations
|
|
Frequency |
Percent |
Valid Percent |
CumulativePercent |
|
|
Valid |
Haemorrhagic stroke |
1 |
2.2 |
2.2 |
2.2 |
|
No |
36 |
78.3 |
78.3 |
80.4 |
|
|
Polyneuropathy |
5 |
10.9 |
10.9 |
91.3 |
|
|
|
Seizure |
4 |
8.7 |
8.7 |
100.0 |
|
Total |
46 |
100.0 |
100.0 |
|
|
Discussion
This study aimed to identify the risk factors predisposing COVID-19 inpatients to develop neurological manifestations. Amongst the 460 COVID-19 ICU inpatients, 46 of them created neurological events. Some risk factors that might influence the potential to establish neurological events are past neurological diseases and chronic non-neurological disorders such as diabetes, hypertension, and cardiovascular risk factors. Other risk factors include demographics such as age, gender, and ethnicity.
One of the significant variables associated with neurological complications was age over 63 years, as indicated by p<0.05, Whereas COVID-19 inpatients with a mean age of 50 years ± 14 did not develop any neurological symptoms. This seems consistent with previous research wherein the study mentions that the risk of developing critical neurologic events is strongly correlated with age above 60. According to previous research, older adults have an increase in age-dependent pro-inflammatory cytokines called inflammation, which increases the expression of CD16 [5].
CD16 is a receptor expressed on Natural Killer cells (NK), and it is found to be the only receptor that can activate a resting NK cell. NK cells are responsible for secreting pro-inflammatory cytokines such as IFN-γ, TNF, and GM-CSF. These pro-inflammatory cytokines activate the innate immune cells, including monocytes and neutrophils, which cause oxidative stress and damage the surrounding tissues, resulting in chronic neuroinflammation, therefore inducing neural damage and neurological manifestations seen in older inpatients.
Previous research has suggested that pre-existing chronic medical conditions such as cardiovascular diseases, hypertension, diabetes, and neurological diseases are strongly associated with COVID-19 inpatients developing neurological consequences. The data showed that amongst the 46 COVID-19 patients who developed neurological manifestations, 34 (73.9%) of them had diabetes. Following the study, the expression of ACE2 is upregulated in the blood vessels of COVID-19 patients with diabetes, therefore increasing the ability of SARS-CoV-2 to enter the blood vessels in the brain and leading to the development of cerebrovascular diseases (CVD).
The second most common comorbidity amongst inpatients in this study who developed neurologic events was hypertension. As mentioned previously, SARS-CoV-2 enters the cell through ACE2. ACE2 is part of the renin-angiotensin system (RAS), which helps regulate the salt/water balance and blood pressure. Different parts of the brain have neuronal ACE2 (nACE2), which plays a faciliatory role in the neurotropism of SARS-CoV-2 [18]. When SARS-CoV-2 enters the host cell through nACE2, it causes a downregulation of ACE2 expression. This leads to an elevation in angiotensin II and, thus, an imbalance in RAS, leading to neurogenic hypertension.
Uncontrolled blood pressure in a thickened, narrowed, or damaged blood vessel leads to hemorrhages or other neurological consequences. However, in the study [12], there isn't any correlation between hypertension and the development of neurological consequences as p >0.05. According to their research, the second most common comorbidity that causes neurological manifestations is hypertension.
The data showed that the third most prevalent underlying risk factor amongst the 46 inpatients with neurological consequences was cardiovascular diseases, as 20 (43.5%) inpatients had it in their past medical history. However, in previous studies, there isn't any correlation between cardiovascular diseases and the development of neurological manifestations as p >0.05 [12].
As studied by (Frontera et al., 2021), the most common type of neurologic event caused by SARS-CoV-2 was toxic/metabolic encephalopathy [15]. However, our data suggests that ischemic stroke has the highest frequency. Our results indicate that the majority of the patients who developed neurological manifestations were South Asians Table 8. This might be due to the discrepancies like the population amongst the studies. For instance, study [15] took place in China, with a relatively homogenous population [16]. However, the UAE's South Asian population is highly prevalent [3]. South Asian countries such as India and Pakistan have the highest prevalence of people who have diabetes [38]. As seen by our data, diabetes plays a significant role in the development of neurological manifestations. The study (Chen, Rong, et al., 2016) discusses that diabetes is an important risk factor for the development of ischemic stroke with poorer clinical outcomes, therefore supporting the data [39].
This research study has strengths as it has analyzed different risk factors that can cause neurological manifestations. This study has used various databases and published articles to reduce research bias. Moreover, in contrast to prior retrospective studies that focused on COVID-19 patients who developed new neurologic disorders, this study includes COVID-19 inpatients who have primary neurological diseases such as epilepsy and that they developed new neurological manifestations during the period they were RT-PCR SARS-CoV-2- positive. It uses the STROBE guidelines to improve the quality of reporting, and lastly, it has an adequate sample size to minimize data unreliability.
Despite this, the paper has some distinct limitations. The data collected was binary. Therefore, outliers couldn't be removed, and the data is subjective to reporting bias. Secondly, numerous inpatients admitted to the ICU were transferred from Dubai Health Authorities (DHA), and their records were missing, so we can't be sure whether they had a neurologic event before moving into Mediclinic Welcare Hospital. Furthermore, the majority of the patients who developed neurological manifestations had severe underlying comorbidities; it is unclear whether these neurological events were triggered by SARS-CoV-2 or whether they would have created one either way. To limit this dilemma, we can conduct a standardized test to compare similar in patients who did not have COVID-19 and determine whether they had any neurological consequences. This will help determine the proportion of COVID-19 patients who developed neurological manifestations from SARS-CoV-2 and those who created it due to their associated comorbidities. Lastly, many inpatients needed health insurance, so their records were available or complete. All these factors influence the reliability of the data collected.
This study can further investigate different biomarkers as risk factors. For instance, we can examine whether elevated D-dimer levels can predispose COVID-19 patients to thrombotic strokes. However, even though D-dimer levels were only collected of COVID-19 inpatients with neurological manifestations (Table 9), we can firmly conclude that these patients had significantly elevated D-dimer levels, with alive patients having a mean of 4107.20ng/mL and dead patients having a mean of 7372.69ng/mL. The usual range of D-dimer should be <500ng/mL [40]. There is a significant difference in the D-dimer levels of both groups, proven by P<0.05. Therefore, in future research papers, we can look at the implication of increased D-dimer levels in COVID-19 patients with and without neurological manifestations to make a conclusive statement regarding the effects of D-dimer levels in COVID-19 inpatients.
Disclaimer
The authors alone are responsible for the views expressed in this article, and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
Study Funding: The authors report no targeted funding.
Disclosure: The authors report no relevant disclosures.
References
- Abel AM, Yang C, Thakar MS, Malarkannan S (2018) Natural killer cells: development, maturation, and clinical utilization. Frontiers in immunology. (9): 1869.
- Agency for Clinical Innovation. ADULT NEUROLOGICAL OBSERVATION CHARTEducation Package.; 2013.
- Blogger GM. United Arab Emirates Population Statistics (2018).
- Bohmwald K, Gálvez NMS, Ríos M, Kalergis AM (2018) Neurologic Alterations Due to Respiratory Virus Infections. Front Cell Neurosci. 12: 386.
- Bossù P, Toppi E, Sterbini V, Spalletta G (2020) Implication of Aging Related Chronic Neuroinflammation on COVID-19 Pandemic. J Pers Med. 10(3): 102.
- Bryant JW, Shariat-Madar Z (2009) Human plasma kallikrein-kinin system: physiological and biochemical parameters. Cardiovasc Hematol Agents Med Chem. 7(3): 234-50.
- Choi JY, Lee HK, Park JH, Cho SJ, Kwon M, et al. (2020) Altered COVID-19 receptorACE2 expression in a higher risk group for cerebrovascular disease and ischemic stroke. Biochemical and Biophysical Research Communications. 528(3): 413-419.
- Chen X, Laurent S, Onur OA, Kleineberg NN, Fink GR, et al. (2021) A systematic review of neurological symptoms and complications of COVID-19. J Neurol. 268(2): 392-402.
- Cooper MA, Fehniger TA, Caligiuri MA. (2001) The biology of human natural killer-cell subsets.Trends in immunology. 22(11): 633-40.
- Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, et al. (2020) Neurological associations of COVID-19. Lancet Neurol. 19(9): 767-783.
- Ennab F, ElSaban M, Khalaf E, Tabatabaei H, Khamis AH, et al. (2021) Clinical Characteristics of Children With COVID-19 inthe United Arab Emirates: Cross-sectional Multicenter Study. JMIR Pediatr Parent. 4(4): e29049.
- Flores-Silva FD, García-Grimshaw M, Valdés-Ferrer SI, Vigueras-Hernández AP, Domínguez-Moreno R, et al. (2021) Neurologic manifestations in hospitalized patientswith COVID- 19 in Mexico City. PLoS One. 16(4): e0247433.
- Ferrucci L, Fabbri E (2018) Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 15(9): 505-522.
- Ferté T, Ramel V, Cazanave C, Lafon ME, Bébéar C, et al. (2021) Accuracy of COVID-19 rapid antigenic tests comparedto RT- PCR in a student population: The StudyCov study. J Clin Virol. 141: 104878.
- Frontera JA, Sabadia S, Lalchan R, et al. (2021) A Prospective Study of Neurologic Disorders in Hospitalized Patients With COVID-19 in New York City. Neurology. 96(4): e575-e586.
- Han E, Paik C (2017) Ethnic integration and development in China. World Development. 93(C): 31-42.
- Ikeda K, Kawakami K, Onimaru H, Okada Y, Yokota S, et al. (2017) The respiratory control mechanisms in the brainstem and spinal cord: integrative views of the neuroanatomy and neurophysiology. JPhysiol Sci. 67(1): 45-62.
- Kulkarni PG, Sakharkar A, Banerjee T (2022) Understanding the role of nACE2 in neurogenic hypertension among COVID-19 patients. Hypertension Research. 45(2): 254-269.
- Loewenstein D, Rabbat M (2021) Neurological complications of systemic hypertension.Handb Clin Neurol. 177: 253-259.
- Lotze MT, Thomson AW (2009) Natural killer cells: Basic science and clinical application. 155-168.
- McKeigue PM, Miller GJ, Marmot MG (1989) Coronary heart disease in south Asians overseas: a review. J Clin Epidemiol. 42(7): 597-609.
- Ntaios G, Michel P, Georgiopoulos G, Guo Y, Li W, et al. (2020) Characteristics and outcomes in patients with COVID-19 and acute ischemic stroke: the global COVID-19 stroke registry. Stroke. 51(9): e254-e258.
- Palladino M (2021) Complete blood count alterations in COVID-19 patients: A narrative review. Biochem Med (Zagreb). 31(3): 030501.
- Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, et al. (2020) Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol. 92(7): 699-702.
- Smith A, Patterson C, Yarnell J, Rumley A, Ben-Shlomo Y, et al. (2005) Which hemostatic markers add to the predictive value of conventional risk factors for coronary heart disease andischemic stroke? The Caerphilly Study. Circulation. 112(20): 3080-7.
- Soler EP, Ruiz VC (2010) Epidemiology and risk factors of cerebral ischemia and ischemic heart diseases: similarities and differences. Curr Cardiol Rev. 6(3): 138-49.
- Spence JD, de Freitas GR, Pettigrew LC, Ay H, Liebeskind DS, et al. (2020) Mechanisms of Stroke in COVID-19. Cerebrovasc Dis. 49(4): 451-458.
- Teuwen LA, Geldhof V, Pasut A, Carmeliet P (2020) COVID-19: the vasculature unleashed.Nat Rev Immunol. 20(7): 389-391.
- Xiong W, Mu J, Guo J, Lu L, Liu D, et al. (2020) New onset neurologic events in people with COVID-19 in 3regions in China. Neurology. 95(11): e1479-e1487.
- Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE et al. (2020) Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: A review. JAMA neurology. 77(8):1018-1027.
- Khatoon F, Prasad K, Kumar V (2020) Neurological manifestations of COVID-19: available evidences and a new paradigm. J Neurovirol. 26(5): 619-630.
- Vymazal J, Rulseh AM, Keller J, Janouskova L (2012)Comparison of CT and MR imaging in ischemic stroke. Insights intoimaging. 3(6): 619-627.
- Tadi P, Lui F (2023) Acute Stroke. StatPearls [Internet].
- Huff JS, Murr N (2023) Seizure. StatPearls [Internet].
- Kumar A, Maini K, Kadian R (2023) Levetiracetam. StatPearls [Internet].
- Jupalli A, Iqbal AM (2022) Enoxaparin. StatPearls [Internet].
- Coull BM, Williams LS, Goldstein LB, Meschia JF, Heitzman D, et al. (2002) Anticoagulants and antiplatelet agents in acute ischemic stroke: report of the Joint Stroke Guideline Development Committeeof the American Academy of Neurology and the American Stroke Association (a division of the American Heart Association). Stroke. 33(7): 1934-42.
- Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates forthe year 2000 and projections for 2030. Diabetes care. 27(5): 1047-53.
- Chen R, Ovbiagele B, Feng W (2016) Diabetes and Stroke: Epidemiology, Pathophysiology, Pharmaceuticalsand Outcomes. Am J Med Sci. 351(4): 380-6.
- Sikora-Skrabaka M, Skrabaka D, Ruggeri P, Caramori G, Skoczyński S, et al. (2019) D-dimer value in the diagnosis of pulmonary embolism-may it exclude only? J Thorac Dis.11(3): 664-672.
- Noro F, de Mendonça Cardoso F, Marchiori E (2021) COVID-19 and Posterior Reversible EncephalopathySyndrome. Neurol Clin Pract. 11(2): e202-e204.
- Veleri S (2022) Neurotropism of SARS-CoV-2 and neurological diseases of the central nervous system in COVID-19 patients. Exp Brain Res. 240(1): 9-25.
- Lallana S, Chen A, Requena M, Rubiera M, Sanchez A, et al. (2021) Posterior reversible encephalopathy syndrome (PRES) associated withCOVID-19. J Clin Neurosci. 108-112.