Paras V1, Sanjeeva V2, Vinay V2, Devika T3, Amit Sharma4 *
1Senior Resident, TB & RD, NITRD
2DNB Resident, TB & RD, NITRD
3HOD, Biochemisry, NITRD
4Chest Physician, NITRD
*Corresponding Author: Amit Sharma, Chest Physician, National Institute of Tuberculosis and Respiratory Diseases (NITRD), near Qutab Minar, Sri Aurobindo Marg, New Delhi – 110030
Abstract
We present an extremely rare case where after occurrence of ATT induced hepatoxicity, Urse-deoxycholic Acid (Udca) was started simultaneously with stopping all the drugs in the ATT which were Isoniazid, Rifampicin, Ethambutol and Pyrazinamide. However, patient's liver enzymes remained high even 20 days after stopping ATT. Once patient presented to us, we stopped Udca and gave no medicine to the patient because the patient had Tubercular Lymphadenothy (R) Cervical and only had complaint of loss of appetite and was otherwise healthy. Post stoppage of Udca, the patient's liver enzymes started to fall and became normal in a month. We reintroduced Isoniazid but patient developed severe headache on the first day and had to go to emergency for relief. Hence, we started Rifampicin which patient tolerated and LFT remained stable. Patient was then started on Rifampicin, Ethambutol and Levofloxacin. Patient's LFT after 2 month is normal and patient has no symptoms and there is almost complete resolution of the Lymphadenopathy.
Ursedeoxycholic acid (Udca) is a bile acid produced in humans as secondary acid by metabolism of intestinal bacteria. It finds clinical application in the treatment of Cholesterol bile stone and naturally occurring bile has been used as medical therapy in gallstone disease (cholelithiasis) and for biliary sludge. Udca helps reduce the cholesterol saturation of bile and leads to gradual dissolution of cholesterol-rich gallstones [1].
Bariatric surgery is another indication for Udca where rapid weight loss predisposes the overproduction of biliary cholesterol along with biliary dyskinesia secondary hormonal changes [2].
It is FDA approved for the treatment of Primary Biliary Cholangitis [3]. It is also prescribed to counteract the Hepato-toxicity of anti-tubercular drugs such as Isoniazid (H), Rifampicin (R) and Pyrazinamide (Z).
We report a case in which Udca was prescribed to alleviate the hepato-toxicity of anti-tubercular therapy but instead led to a paradoxical progressive rise in Liver enzymes which subsided once Udca was stopped. With this unique case, we want to draw attention to the indiscriminate use of Udca in conditions in which it is probably not effective and in rare cases may even be harmful.
Keywords: Drug-induced, Tuberculosis, hepato-protective, cirrhosis
Case report
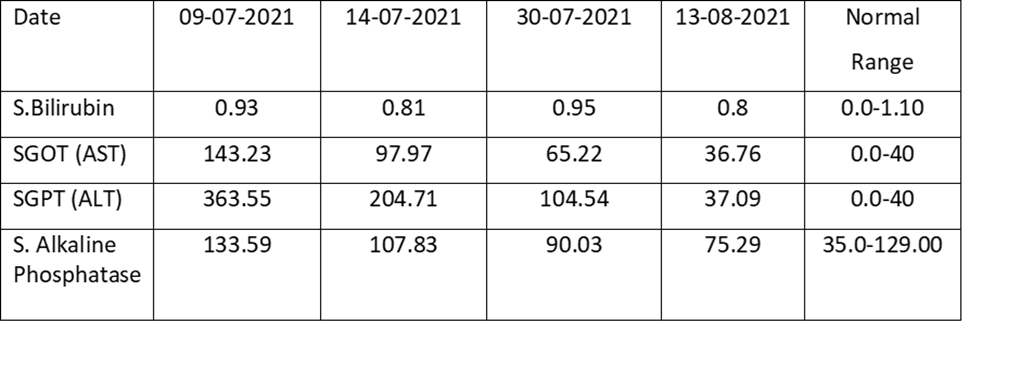
A young lady of 30 years presented to the Outpatient Department (OPD) of the National Institute of Tuberculosis and Respiratory Diseases (NITRD) on 8th of July 2021. The patient had been diagnosed with Right Tubercular Cervical Lymphadenopathy and started on Anti-tubercular therapy (ATT) consisting of Isoniazid (H), Rifampicin (R), Ethambutol (E) and Pyrazinamide (Z) on 15th May 2021 which was stopped after a month around mid-June due to symptomatic elevation of Liver enzymes. However, even after cessation of ATT, the patient’s liver enzymes showed a slightly rising trend and when she presented to us, we got a Liver Function Test at our Institute. We also got HbsAg, Anti-HCV, antibodies against HAV and HEV and HIV testing all of which came out to be negative. Ultrasonography of the abdomen did not reveal any anomaly and the Liver was normal as per the report. It was felt that the reason of the rising trend in the Liver Enzymes could be the Udca which was being given to the patient. UDCA was stopped. No drugs were given to the patient. The table below shows the steady decrease in the liver enzymes after stoppage of Udca w.e.f. 9th of July 2021 to August 13th August 2021. On starting Isoniazid patient complained of itching and severe headache and hence it was stopped only after 1 dose. Rifampicin was re-introduced and the patient tolerated the medicine well and her LFT came normal after 8 days of Rifampicin. The regime was thus made of Rifampicin, Ethambutol and Levofloxacin and after 2 months of treatment patient is asymptomatic and there is almost complete resolution of Lymphadenopathy.
Table: Illustrating the fall of Liver enzymes of the patient after stopping Udca
Discussion
Udca is a hepato-protective agent and as mentioned above and finds place in the management of dissolution of cholesterol gall stones and sludge. The treating physician mostly prescribes Udca in management of Drug-induced hepatitis due to H, R and Z in ATT. No agent including Udca has been conclusively shown to have a definite hepato-protective action in mitigating ATT-induced hepatotoxicity [4]. However, there is also some tentative evidence which has provided a possible hepato-protective role of Udca in ATT induced Liver injury [5]. However, this case is unique because it showed elevation of Liver Enzymes which rapidly normalized after cessation of Udca. There is some evidence that Udca may lead to an increase in Liver Enzymes in advanced liver diseases with cirrhosis leading to clinical decompensation. Hence giving Udca to patients with advanced liver disease may be inimical to these subgroups of patients [6].
In our case, patient’s ultrasound was absolutely normal with no evidence of parenchymal involvement. The importance of this case lies in the fact that even a patient with a normal liver can develop hepato-toxicity due to Udca. We could not find any other literature which highlights the paradox of hepato-toxicity by this hepato-protective drug in patients with normal liver.
To conclude, a patient who is having elevated liver enzymes, especially after stopping ATT, a detailed drug history has to be taken and in case of Udca administration, a high index of suspicion needs to be maintained to rule out Udca as the drug causing hepato-toxicity.
References
- Jungst C, Kullak-Ubilickk GA, Jungst D (2006) Gallstone disease: Microlithiasis and sludge. Best Practice & Research. Clinical Gastroenterology. 20(6): 1053–62.
- Magouliotis DE, Tasiopoulou VS, Svokos AA, Svokos KA, Chatedaki C, et al. (2017) Ursodeoxycholic Acid in the Prevention of Gallstone Formation After Bariatric Surgery: an Updated Systematic Review and Meta-analysis. Obesity Surgery. 27(11): 3021–3030.
- Bowlus CL, Kenney JT, Rice G, Navarro R (2016) Primary Biliary Cholangitis: Medical and Specialty Pharmacy Management Update. Journal of Managed Care & Specialty Pharmacy. 22 (10-a-s Suppl): S3–S15.
- Xu L, Zhang F, Xu C, Liu KG, Wu W, et al. (2017) Is the Prophylactic Use of Hepatoprotectants Necessary in Anti-Tuberculosis Treatment? Chemotherapy. 62(5): 269-278.
- Asgarshirazi M, Shariat M, Dalili H, Keihanidoost Z (2015) Ursodeoxycholic Acid Can Improve Liver Transaminase Quantities in Children with Anticonvulsant Drugs Hepatotoxicity: A Pilot Study. Acta Medica Iranica, 53(6): 351-355.
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Ursodiol (Ursodeoxycholic Acid) [Updated 2017 Sep 25].



