Ndjekembo Shango D*, Vanye T, Simonet O, Vallot F
Intensive Care Department, Centre Hospitalier de Wallonie-Picarde, Tournai, Belgium
*Corresponding Author: Ndjekembo Shango D, Intensive Care Department, Centre Hospitalier de Wallonie-Picarde, Tournai, Belgium
Abstract
Background: Spontaneous pneumomediastinum (SP) is the presence of free air into extra-alveolar tissues within the mediastinum, without notion of trauma. This is a rare condition that may occurs as a complication in some severe acute respiratory failure in SARS-CoV-2 pneumonia. This complication is more frequently found in patients with severe SDRA. The treatment is essentially conservative.
Case description: We describe the case of a COVID-19 infected patient suffering from severe SP during the pandemic in Belgium.
Discussion: The purpose of this report is to discuss the pathophysiological mechanism, the diagnostic and the management of this rare complication.
Conclusion: SP is a rare and serious complication of SARS-CoV-2 infection. The pathological pathways remains unclear. It doesn’t seem to be associated with an higher rate of mortality. The treatment is essentially conservative.
Keywords: Spontaneous pneumomediastinum, Covid-19, SARS-CoV-2, SDRA
Introduction
SARS-CoV2 pneumonia is due to an infection by a new strain of coronavirus. This virus was first detected in Wuhan, China, in December 2019. It spread quickly throughout that region, and the world created a pandemic responsible for a significant sanitary crisis in most countries [1]. At the end of 2021, over 250 million cases were identified worldwide, with over 5 million deaths. [2] The classic clinical symptoms of SARS-CoV-2 pneumonia are cough, fatigue, muscle aches, headaches, and digestive problems. Dyspnea is the most common symptom in severe cases, typically associated with hypoxemia [3]. 5-20 % of these patients progressively deteriorate towards respiratory distress and develop acute respiratory distress syndrome, defined by the sudden appearance of bilateral lung infiltrations, pulmonary edema, and hypoxemia.[4]
Pneumothorax is a rare complication, occurring in 1 % of SARS- CoV2 patients [5], with pneumomediastinum reported in a small number of patients [6-9]. The exact pathophysiological mechanism responsible for this complication remains unclear.
We here review the case of a 49-year-old patient with SARS-CoV2 pneumonia, complicated with spontaneous pneumomediastinum.
Description
A 49-year-old male attended the Emergency Department (ED) with a 10-day history of dyspnea and cough.
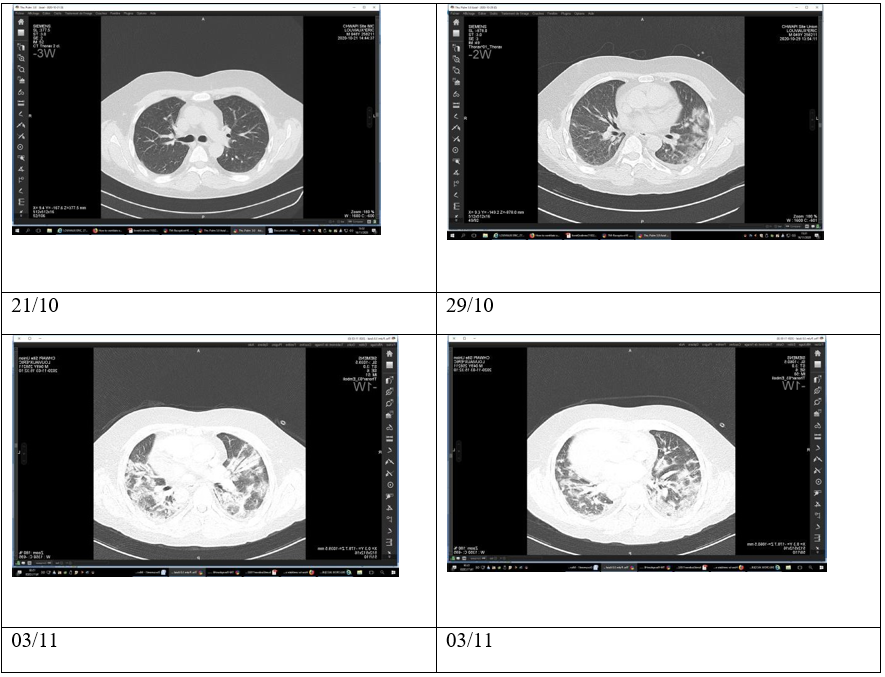
A chest CT scan, performed at the beginning of the symptoms, had been interpreted as regular (figure 1, 29/10 ). He had a significant medical history, including hypertension and kidney transplantation, requiring ongoing immunosuppression with tacrolimus 4mg, mycophenolate mofetil 2g, and methylprednisolone 4mg. The patient was not known to smoke or consume drugs or alcohol.
On arrival at ED, the vital signs were within normal ranges, with blood pressure 140/97 mm < hg, heart rate 92/min, and capillary oxygen saturation level of 93 %. The patient was afebrile and hemodynamically stable but displayed significant dyspnea with increased work of breathing and increased respiratory of 36/min. Oxygen therapy was initiated via a non-rebreathing mask.
Laboratory investigations showed a modest inflammatory syndrome with a CRP of 11.8 mg/L (ref <5mg/L), an average white blood cell count at 4.12x10/microL (ref 3.7 -9.5 x10/microL), associated with lymphopenia at 1.6x10/microL (ref 12-47x10/micro). LDH was mildly elevated at 261U/L (ref 87-241U/L). His renal function was considered stable, with an eGFR of 40mL/min/1.73m2).
Arterial blood analysis demonstrated pH 7.43, pCO2 35mmHg, pO2 71mmHg, SpO2 95 %, and bicarbonates 23mmol/L.
The diagnosis of SARS-CoV2 was confirmed by RT-PCR (Reverse Transcriptase polymerase-chain reaction) on the nasopharyngeal sample, and the patient was admitted to the COVID ward. Treatment was immediately started with Dexamethasone 6mg/day and Enoxaparin 40mg 2x/day.
A thoracic CT scan on Day 2 of admission showed bilateral consolidation affecting 20% of the pulmonary parenchyma. No pneumothorax or pneumomediastinum was detected on this exam (figure 29/10).
On Day 6 of admission, the patient suddenly deteriorated, displaying grade III dyspnea on the mMRC scale without chest pain and requiring increasing supplemental oxygen therapy with 4L/min O2 to maintain the peripheral blood oxygen saturation level at 93 %. A repeat thoracic CT scan demonstrated increased pulmonary consolidation, ruling out pulmonary embolism (figure 3/11). The patient rapidly deteriorated, requiring 15L/min O2 via reservoir mask, and was admitted to the Intensive Care Unit (ICU) with a Rox index of 7.61. Upon initiation of Oxygen therapy with Optiflow (FiO2 90 %, debit 60L/min), the patient clinically improved. Antibiotic treatment was also started with Piperacillin-tazobactam and azithromycin.
On Day 10, his respiratory status suddenly worsened, requiring intubation and ventilated with the following parameters: PCV Fi02 70 %, Pinsp12, PEEP 10, VT 450, FR 18, FR spot 21, Sp02 96 %, and P/F 134.
The patient remained stable until day 13, when he developed critical subcutaneous emphysema in the thorax and neck region. A chest x- ray confirmed the extent of the subcutaneous emphysema.
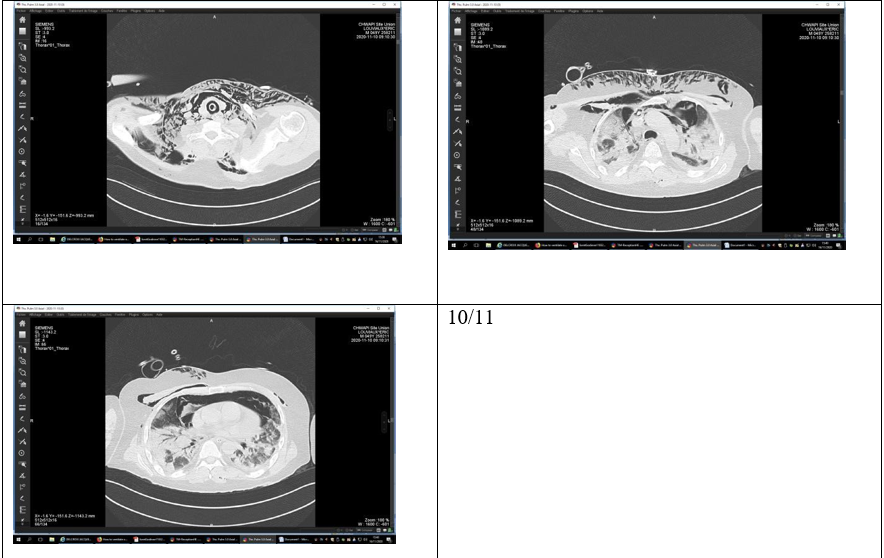
Bronchial microscopy did not identify any lesions that could explain this emphysema. A fourth thoracic CT scan showed that 75 % of the pulmonary parenchyma was affected and that there was a large pneumomediastinum with an extensive extension of the sub- cutaneous emphysema without a pneumothorax (figure 10/11).
After a pluri-disciplinary discussion, the patient was transferred to the Operating Theater for a right lateral mediastinotomy, and a drain was left in place.
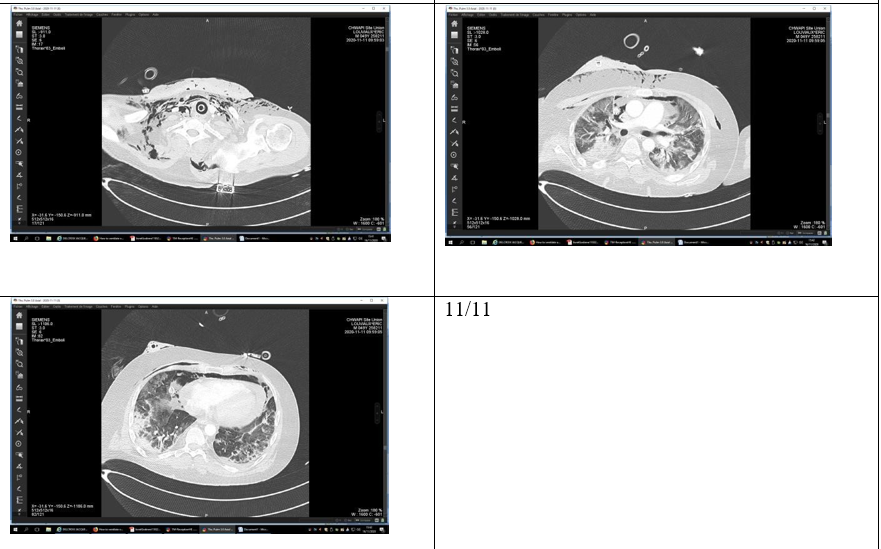
There was subsequently an improvement clinically and radiologically (figure 11/11).
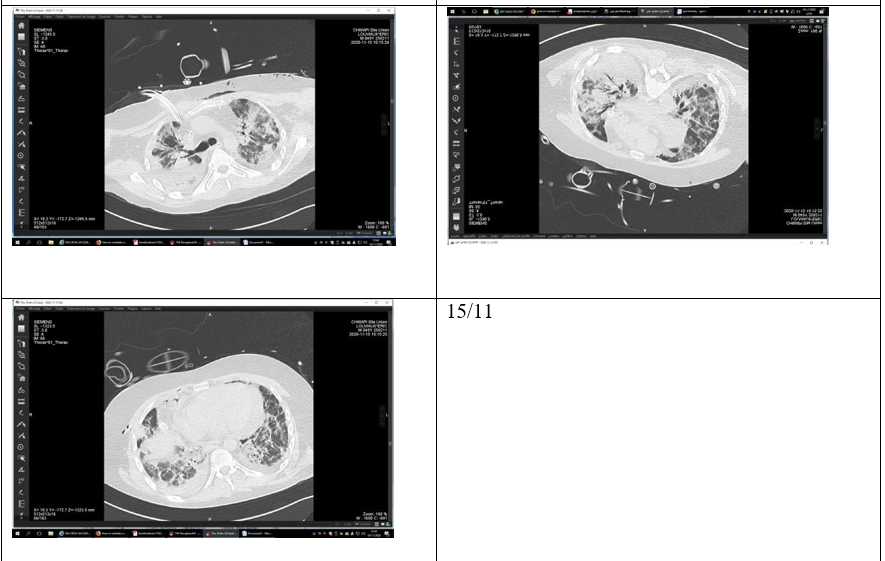
The patient had several episodes of acute hypoxemia during days 15 &16, ultimately requiring ventral position ventilation (figure 13/11). A thoracic CT scan on Day 18 (figure 15/11) showed a hyperdense pericardial effusion of 25mm and worsening pulmonary consolidation.
Upon improvement, the patient was extubated on Day 20, but 10 hours after extubation, he developed acute respiratory distress with severe hypoxemia associated with hypotension, refractory to vasopressor agents.
The patient had two cardiac arrests, with zero no-flow and a rapid recuperation of a spontaneous cardiac rhythm.
During reanimation, the patient was re-intubated. Upon developing major ARDS, ventilation became challenging due to limited pulmonary compliance with high respiratory resistance. Severe respiratory acidosis occurred, and the patient died the following day.
Iconographies
Discussion
Spontaneous pneumomediastinum is a rare complication where one notes the presence of air in the mediastinum with an identifiable cause. This condition was officially described in 1939 by Dr. Louis Hamman, but it has been known since 1819 thanks to the studies of Dr. René Laennic. [12] It occurs more frequently in young males.
Smoking, drug abuse, or pre-existing pulmonary diseases are risk factors. (Table 1) Pneumomediastinum is a known complication of positive pressure ventilation. [11] Common clinical signs are dyspnea, chest pain, neck pain, cough and odynophagia (Table 2).
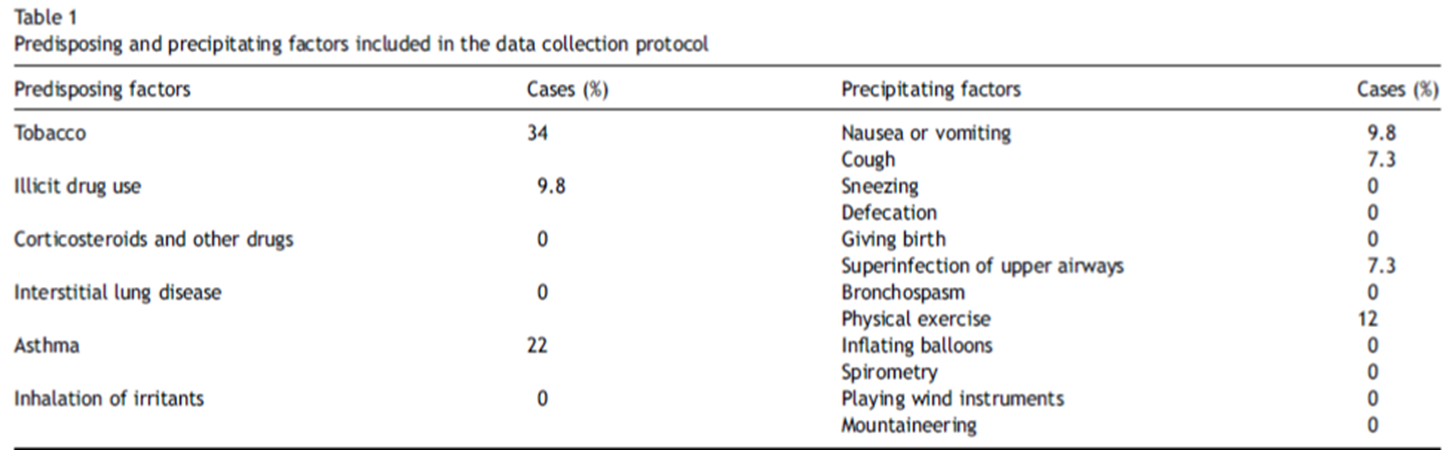
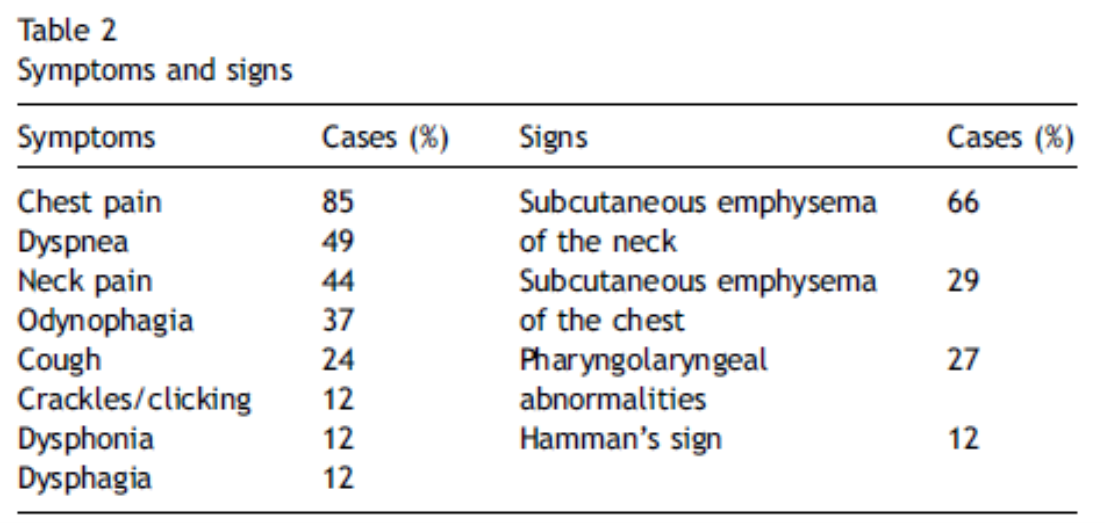
Tables from 11
SARS-CoV2 pneumonia can be responsible for atypical pneumonia evolving into ARDS. Rare cases of pneumomediastinum have been reported in COVID-19 patients. [6,10 &13,15]. Spontaneous pneumomediastinum associated with viral pneumonia has been described in children during the H1N1 epidemic. Some cohorts reported up to 11 % of spontaneous pneumomediastinum during the SRAS outbreak in 2003. [19,20] Typically, 3 mechanisms are responsible for pneumomediastinum. The gas can be produced by germs infecting the mediastinum, which can originate from either an esophageal (traumatic or not) or tracheal perforation. A pressure difference between the intra-alveolar pressure and the interstitial pulmonary pressure can cause it. The intra-alveolar pressure is increased during a Valsalva maneuver (coughing, vomiting, birth, and sneezing), whereas extreme respiratory efforts, diabetic acidosis, marijuana use, and sudden depressions in the atmospheric pressure can decrease the interstitial pressure. [10,11] Most reported cases of pneumomediastinum are in patients with ARDS, which is a risk factor independent of the barotraumatic effects of mechanical ventilation. High tidal volumes associated with severe lung infections are dangerous in COVID-19 patients. Ventilating ARDS patients must be prudent with a tidal volume of 4-6 mL/Kg IBW and a pressure plateau of less than 30 cmH20. Using APRV does not provide an advantage over other types of ventilation in ARDS patients and should be reserved for less severe patients. [16,17]
The physiopathological mechanism responsible for creating a pneumomediastinum is “The Macklin Effect,” first described in 1944 by Dr. Macklin.
It begins with an endo-bronchial pressure increase, thus increasing the pressure gradient between the alveolus and the pulmonary parenchyma, ultimately resulting in alveolar rupture releasing air in the peri-bronchial space, which will travel until reaching the mediastinum. [11]
The exact mechanism of pneumomediastinum in COVID-19 pneumonia patients must be clearly understood. There is a hyperinflammatory state which can favor this condition. Different tools are hypothetically incriminated: interstitial lymphocytic infiltration vs. a systematic involvement of alveolar spaces, which eventually causes alveolar rupture; however, further studies are needed to confirm these notions. [18]
The literature indicates that when pneumomediastinum appears in COVID-19 patients, it occurs after a mean of 17.25 days, counting from the beginning of the symptoms. [13] A 2004 study of ARDS patients demonstrated pneumomediastinum after a mean of 19.6 days. [20] Our patient had his pneumomediastinum after only 13 days. The gold standard to diagnose a pneumomediastinum is the CT scan. [11] A chest x-ray can sometimes be sufficient if it includes both posteroanterior and lateral views.
The treatment is, of course, targeted at the causal mechanism. However, supportive therapy, including rest, pain control, and oxygen administration, can typically already achieve clinical improvement in 75 % of patients. [11]
Conclusion
Pneumomediastinum is a rare complication in patients with SARS- CoV-2 pneumonia. These patients are at higher risk for this specific complication due to their hyperinflammatory state and the fact that some are in ARDS. Typically, this complication appears 2-3 weeks after the symptoms start. The treatment is supportive, but when possible, the cause should be identified (to be treated eventually). This rare complication may have severe consequences, mainly when unrecognized, so the clinician must remain attentive to detect it as early as possible.
References
- World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19 — 11 March 2020.
- Johns Hopkins University Coronavirus Resource Center. COVID- 19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. 2020.
- Wang D, Hu B, Hu C, Zhu F, Liu X, et al. (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 323(11): 1061-1069.
- Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. (2012) Acute respiratory distress syndrome: the Berlin Definition. JAMA. 307(23): 2526-2533.
- Chen N, Zhou M, Dong X, Qu J, Gong F, et al. (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 395(10223): 507–513.
- Wang W, Gao R, Zheng Y, Jiang L (2020) COVID-19 with spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema. J Travel Med. 27(5): taaa062.
- Wang J, Su X, Zhang T, Zheng C (2020) Spontaneous pneumomediastinum: a probable unusual complication of coronavirus disease 2019 (COVID-19) pneumonia. Korean J Radiol. 21(5): 627–628.
- Sun R, Liu H, Wang X (2020) Mediastinal emphysema giant bulla, and pneumothorax developed during the course of COVID- 19 pneumonia. Korean J Radiol. 21(5): 541–544.
- Zhou C, Gao C, Xie Y, Xu M (2020) COVID-19 with spontaneous pneumomediastinum. Lancet Infect Dis. 20(4): 510.
- Kolani S, Houari N, Haloua M, Alaoui Lamrani Y, Boubbou M, et al. (2020) Spontaneous pneumomediastinum occurring in the SARS-COV-2 infection. IDCases. 21: e00806.
- Macia I, Moya J, Ramos R, Morera R, Escobar I, et al. (2007) Spontaneous pneumomediastinum: 41 cases. Eur J Cardiothorac Surg. 31(6): 1110–4.
- Hamman L. Spontaneous mediastinal emphysema. Bull Johns Hopkins Hospital 1939;64:1—21.
- Gorospe L, Ayala-Carbonero A, Ureña-Vacas A, Fra Fernández S, Muñoz-Molina GM, et al. (2020). Spontaneous Pneumomediastinum in Patients With COVID-19: A Case Series of Four Patients. Neumomediastino espontáneo en pacientes con COVID-19: una serie de cuatro casos. Archivos de bronconeumologia. 56(11): 754–756.
- Loffi M, Regazzoni V, Sergio P, Martinelli E, Stifani I, et al. (2020). Spontaneous pneumomediastinum in COVID-19 pneumonia. Monaldi Archives for Chest Disease. 90(4).
- Mimouni H, Diyas S, Ouachaou J, Laaribi I, Oujidi Y, et al. (2020) Spontaneous Pneumomediastinum Associated with COVID-19 Pneumonia", Case Reports in Medicine. 2020: 4969486.
- Carsetti A, Damiani E, Domizi R, Scorcella C, Pantanetti S, et al. (2019) Airway pressure release ventilation during acute hypoxemic respiratory failure: a systematic review and meta- analysis of randomized controlled trials. Annals of intensive care. 9(1): 44.
- Eisner MD, Thompson BT, Schoenfeld D, Anzueto A, Matthay MA (2002) Airway pressures and early barotrauma in patients with acute lung injury and acute respiratory distress syndrome. Am J Respir Crit Care Med. 165(7): 978-82.
- Copin MC, Parmentier E, Duburcq T, Poissy J, Mathieu D, et al. (2020). Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive care medicine. 46: 1124–1126.
- Luis BAL, Navarro AO, Palacios GMR (2017) Pneumomediastinum and subcutaneous emphysema associated with influenza A H1N1 virus. Lancet Infect Dis. 17(6): 671.
- Chu CM, Leung YY, Hui JY, Hung IF, Chan VL, et al. (2004) Spontaneous pneumomediastinum in patients with severe acute respiratory syndrome. Eur Respir J. 23(6): 802-4.



