Ozge Sonmez1, Tugrul Elverdi2, Tuba Ozkan Tekin2, Selin Kucukyurt Kaya2, Suat Hilal Akı3, Ayse Salihoglu2*
1Department of Internal Medicine, Cerrahpasa Medical Faculty, Istanbul University-Cerrahpasa
2Division of Hematology, Department of Internal Medicine, Cerrahpasa Medical Faculty, Istanbul University-Cerrahpasa
3Department of Pathology, Cerrahpasa Medical Faculty, Istanbul University-Cerrahpasa
*Corresponding Author: Ayse Salihoglu, Associate Professor of Hematology Istanbul University-Cerrahpasa, Cerrahpasa Faculty of Medicine, Department of Internal Medicine, Division of Hematology, Istanbul/Turkey.
Abstract
Background
Primary plasma cell leukemia (pPCL) is a rare and highly aggressive plasma cell disorder. Extreme leukocytosis is an infrequent finding in pPCL.
Case Presentation
We reported an 82-year-old Romanian female patient who presented with marked leukocytosis (61.9×109/L) and was diagnosed with pPCL. Plasma cells were found to have t(14;20) and 1q21 amplification. Following partial response after two cycles of bortezomib/dexamethasone combination, central nervous system (CNS) relapse occurred. Due to the advanced age and frailty no further therapy could be administered.
Conclusion
She had a fulminant disease course and died within one month of the CNS involvement. PCL should be included in the differential diagnosis of leukocytosis.
Keywords: Plasma cell leukemia, extreme leukocytosis, t(14;20), 1q21 amplification, central nervous system involvement
Introduction
Background
Plasma cell leukemia (PCL) is a rare and highly aggressive plasma cell malignancy. PCL can arise as leukemic progression of pre- existing multiple myeloma (MM) typically at the advanced and late stage (secondary PCL (sPCL)) or develop as de novo (primary PCL (pPCL)). PCL is defined as the presence of circulating plasma cells greater than 20% if there is less than 10×109/L leukocyte in peripheral blood or more than 2×109/L circulating plasma cells in the presence of > 10×109/L leukocytes in peripheral blood. [1,2] Based on the results of recent studies a revision on diagnostic criteria for PCL was suggested. Presence of ≥ 5% plasma cells in the peripheral blood had a similar survival rate and prognostic outcome as classically defined PCL and this cutoff is now used for definition of PCL. [3,4]. Bone marrow failure, extramedullary involvement, renal impairment, elevated lactate dehydrogenase (LDH) and beta-2-microglobulin levels and high risk genetic events are more frequent in PCL compared to MM [5].
We report the case of an 82-year-old female who presented with marked peripheral blood leukocytosis and was diagnosed with both t(14;20) and 1q21 amplification positive pPCL followed by a relapse in the central nervous system (CNS) after a short period of response.
Case Presentation
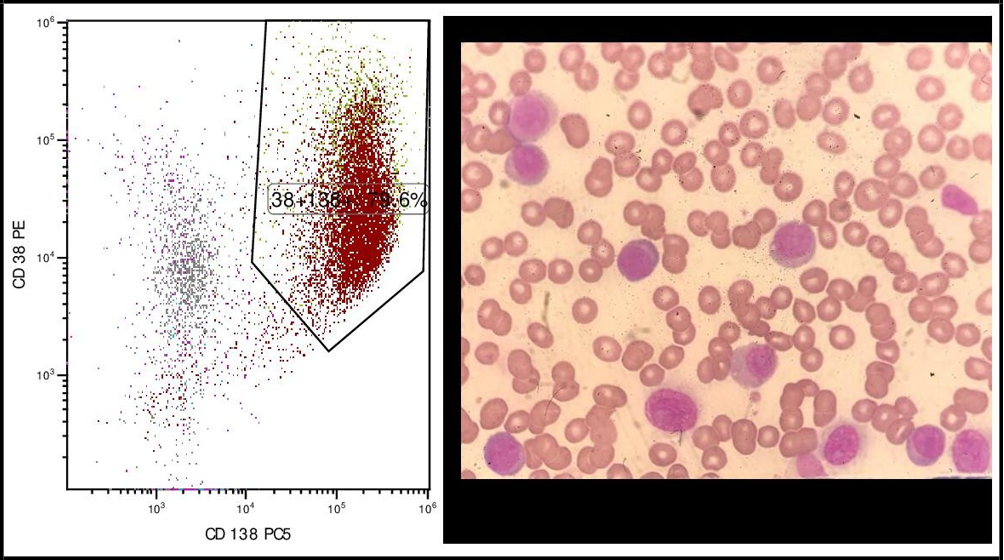
An 82-year-old Romanian female with a history of hypertension, atrial fibrillation and chronic liver disease presented to emergency department with recent onset fatigue and left hip pain. She was a nonsmoker and consumed alcohol regularly for fifteen years. Complete blood count revealed a hemoglobin of 105 g/L, white blood cell (WBC) 61.9 ×109/Land platelets 86 ×109/LSerum protein electrophoresis showed a monoclonal band in the gamma region and immunofixation revealed immunoglobulin G (IgG) kappa isotype. The Table 1 summarizes laboratory test results and reference values. Peripheral blood smear (PBS) showed plasma cells constituting 70% of total leukocytes (Figure 1). Flow cytometric analysis confirmed the expression of CD138 and kappa light chain in these plasma cells whereas CD19 and C56 were found to be negative (Figure 1). Bone marrow aspiration revealed 84% kappa light chain restricted, CD38, CD138 and MUM1 expressing plasma cells negative for CD20 and CD56. Fluorescent in situ hybridization FISH) of bone marrow plasma (cells identified t(14;20), 1q amplification and monosomy 13. Positron emission tomography- computed tomography (PET-CT) imaging showed multiple bulky conglomerated, hypermetabolic lymph nodes at both infra and supradiaphragmatic stations and increased diffuse metabolic uptake in the axial skeletal system. Hydronephrosis secondary to the compression of bulky lymph nodes to the ureter was present on the left side (Figure 2). She was diagnosed with IgG kappa pPCL (ISS and R-ISS stage III).
Her Eastern Cooperative Oncology Group (ECOG) performance status was grade 4. A doublet therapy including bortezomib and dexamethasone was initiated due to the advanced age and frailty.
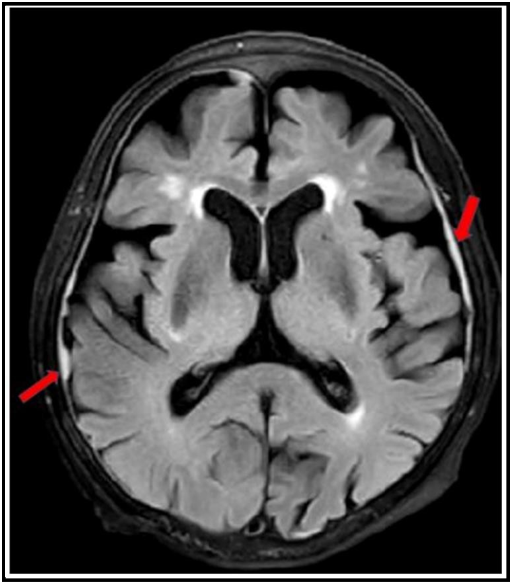
After 2 cycles, the patient achieved partial remission (Table 1). Additionally, both infra- and supradiaphragmatic lymph nodes and hydronephrosis regressed on CT. After one month, she presented with walking difficulty. Cranial magnetic resonance imaging (MRI) demonstrated diffuse nodular thickening of the dura mater (Figure 3). Thoracic and lumbar MRI showed meningeal enhancement compatible with leptomeningeal involvement. Cerebrospinal fluid flow cytometry analysis detected kappa clonal plasma cells. Plasma cells reappeared in the peripheral blood. No further therapy for the full-blown disease progression could be applied due to the general status of the patient and she passed away shortly afterwards.
Table 1: Laboratory test results at diagnosis and during the course of the disease (NA: not available, Ig: immunoglobulin)
|
|
Diagnosis |
After 2 cycles of |
Progression |
Reference Range |
|
Leukocyte (109/L) |
61.9 |
6.4 |
12.4 |
4.3–10.3 |
|
Hemoglobin (g/L) |
105 |
110 |
110 |
120–16 0 |
|
Platelet (109/L) |
86 |
120 |
52 |
156–373 |
|
Creatinine (µmol/L) |
327.08 |
119.34 |
210.39 |
44.20 –79.56 |
|
Calcium (mmol/L) |
2.10 |
2.25 |
1.74 |
2.10–2.55 |
|
LDH (IU/L) |
1454 |
363 |
1366 |
< 250 |
|
Albumin ( g/L) |
30.60 |
37.60 |
25.90 |
35.00-52.00 |
|
Βeta-2 microglobulin (nmol/L) |
3337.57 |
956.74 |
NA |
67.85-186.59 |
|
Serum free kappa light chain (mg/l) |
5060 |
619 |
NA |
3.30-19.40 |
|
Serum free lambda light chain (mg/l) |
17 |
6.43 |
NA |
5.7-26.3 |
|
Kappa/lambda ratio |
297.65 |
96 |
NA |
0.26-1.65 |
|
IgG (g/L) |
51.47 |
19.49 |
37.64 |
7.00–16.00 |
|
IgA (g/L) |
0.37 |
0.34 |
0.01 |
0.7–4.00 |
|
IgM (g/L) |
0.09 |
0.10 |
0.03 |
0.4–2.3 |
Figure 1: Peripheral blood smear(A) and flow cytometry (B and C) of the patient at the diagnosis
Figure 2: PET/CT scan of the patient at the diagnosis
Figure 3: Cranial magnetic resonance imaging of the patient. Red arrows show diffuse nodular thickening of dura mater indicating leptomeningeal involvement
Discussion and Conclusion
PCL should be kept in mind in case of leukocytosis. Only less than 1% of the patients with extreme leukocytosis (> 50×109/L) are diagnosed with PCL [6]. Therefore, our patient had a rare presentation of PCL.
Presence of plasma cells in peripheral blood does not always suggest PCL. Marked peripheral blood polyclonal plasmacytosis mimicking PCL can occur in reactive conditions such as viral infections, autoimmune disease, serum sickness or even angioimmunoblastik T- cell lymphoma. In those conditions, plasma cells do not have kappa or lambda light chain restriction and typically disappear with appropriate treatment of the underlying condition [7,8].
Peripheral blood morphologic analysis and flow cytometry are critical for the correct diagnosis [9]. Flow cytometric analysis of PCL usually reveals CD38 and CD138 positivity. HLA-DR, CD117, CD56 are less frequently expressed in PCL compared to MM whereas CD20 and CD19 are more frequently positive in pPCL [10].
Many cytogenetic abnormalities have been demonstrated in PCL. However, none of them are specific to PCL. Immunoglobulin heavy chain (IgH) translocations including t(4;14), t(11;14) and t(14;16), 8q24 rearrangements, TP53 inactivation, K-RAS and N-RAS mutations, deletion of chromosome 17, deletion of chromosome 13, 1p loss or lq gains and monosomy 13 have been defined in PCL [1,11,12]. The t(14;20) is seen less frequently in PCL [13]. On the other hand, t(14;20) has been associated with poor prognosis and higher prevalence of lytic lesions, renal dysfunction and increased urine monoclonal protein in MM [14]. Murase et al. reported one t(14;20) positive patient with PCL who had CNS involvement. They suggested that t(14;20) positivity might be associated with CNS involvement in PCL [15]. We would like to highlight that our patients’ poor prognosis with extreme leukocytosis and CNS involvement might be due to this high cytogenetic risk burden.
Patients with PCL have an increased tendency to extramedullary spread including CNS which has an ominous prognosis [16,17]. CNS involvement occurs via hematogenous dissemination in PCL. Marked peripheral blood plasma cells may precede CNS involvement [18]. Clinical findings may include cranial nerve palsies, cerebral dysfunction and spinal radiculopathies. MRI and CSF examination are crucial in the diagnostic workup [19,20]. There is no established treatment for PCL with CNS involvement. Therapies including combinations of systemic dexamethasone and pomalidomide and intrathecal methotrexate, methylprednisolone and cytarabine have been reported in the literature [16].
In the multicenter, retrospective study Pagano et al. evaluated 73 pPCl patients. Stage III disease was found in 57 patients. Beta-2 microglobulin was elevated in all patients and LDH was increased in more than half of the cohort. The median leukocyte count was reported to be 13.7×109/L (range 1.3-56.7×109/L). Additionally, ten of the patients (14%) had extramedullary involvement and 4% had lymphadenopathies. High-risk cytogenetic features were observed in 56% of the patients. Bortezomib was given to 7 patients. Lack of response to induction, hypercalcemia and hypoalbuminemia at diagnosis negatively impacted survival while patients who underwent transplantation had better outcomes [21]. The presented case had a much higher leukocyte count and similar characteristics including ISS III disease, high LDH levels, extramedullary involvement and a lack of treatment response. Unfortunately stem cell transplantation was not an option for her because of the age.
The patient did not develop tumor lysis syndrome (TLS) following initiation of treatment despite extreme peripheral plasmacytosis, massive bone marrow infiltration and conglomerated bulky lymph nodes which predisposed the patient to an increased risk of TLS. Zhou Yu and colleagues reported a patient with PCL and extreme leukocytosis (WBC count 125.2×109/L) who presented with spontaneous TLS [22].
The patient responded to bortezomib based induction therapy initially but had an early systemic relapse with meningeal involvement. The optimal therapeutic approach to pPCL is controversial and response durability is brief unless consolidated with high dose melphalan and autologous transplantation followed by maintenance therapy [23]. The patient was ineligible for additional therapies due to advanced age and frailty and had a fulminant disease course. She died within one month of the CNS involvement.
PCL is a rare cause of extreme leukocytosis and clinical awareness of PCL is needed in patients with leukocytosis. Extramedullary involvement including CNS is quite common in PCL. The t(14;20) positivity might be additionally associated with CNS involvement and extreme leukocytosis in the presented patient. CNS involvement may be challenging to diagnose due to heterogeneous symptom burden. Exclusion of frequent neurologic complications caused by myeloma or treatment side-effects is necessary. Treatment is challenging in PCL with CNS involvement especially in patients with poor performance and advanced age.
List of Abbreviations
CNS: central nervous system
IgG: immunoglobulin
GLDH: lactate dehydrogenase
MM: multiple myeloma
PCL: plasma cell leukemia
PET-CT: Positron emission tomography-computed tomography
TLS: tumor lysis syndrome
WBC: white blood cell
Declarations
Ethical approval and consent to participate: Not applicable
Consent for publication: Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written informed consent is available for review by the Editor-in-Chief of this journal.
Availability of data and statement: All data generated or analyzed during this study are included in this article.
Competing interests: The authors have no conflict of interests to declare
Funding statements: This study was not supported by any funding
Authors’ Contributions
1. Conception or design, or analysis and interpretation of data, or both.
2. Drafting the article or revising it.
3. Providing intellectual content of critical importance to the work described.
4. Final approval of the version to be published.
Ozge Sonmez: 1,2,3,4
Tugrul Elverdi:1,3,4,
Tuba Ozkan Tekin:1,4
Selin Kucukyurt Kaya:1,4,
Suat Hilal Akı:3,4,
Ayse Salihoglu: 1,2,3,4,
Acknowledgement: Not applicable
References
- Tiedemann RE, Gonzalez-Paz N, Kyle RA, Santana-Davila R, Price-Troska T, et al. (2008) Genetic aberrations and survival in plasma cell leukemia. 22(5): 1044–1052.
- Gertz MA (2007) Managing plasma cell leukemia. Leuk Lymphoma. 48(1): 5-6.
- Ravi P, Kumar SK, Roeker L, Gonsalves W, Buadi F, et al. (2018) Revised diagnostic criteria for plasma cell leukemia: results of a Mayo Clinic study with comparison of outcomes to multiple myeloma. Blood Cancer J. 8(12): 116.
- Fernández de Larrea C, Kyle R, Rosiñol L, Paiva B, Engelhardt M, et al. (2021) Primary plasma cell leukemia: consensus definition by the International Myeloma Working Group according to peripheral blood plasma cell percentage. Blood Cancer J. 11(12): 192.
- Tuazon SA, Holmberg LA, Nadeem O, Richardson PG (2021) A clinical perspective on plasma cell leukemia; current status and future directions. Blood Cancer J. 11(2): 23.
- Hugo ZP, Perry MC, Steensma DP (2008) Extreme leukocytosis in the 21st Century is often iatrogenic. Blood. 112(11): 5401.
- Touzeau C, Pellat-Deceunynck C, Gastinne T, Accard F, Jego G, et al. (2007) Reactive plasmacytoses can mimick plasma cell leukemia: therapeutical implications. Leuk Lymphoma. 48(1): 207–208.
- Sokol K, Kartan S, Johnson WT, Alpdogan O, Nikbakht N, et al. (2019) Extreme Peripheral Blood Plasmacytosis Mimicking Plasma Cell Leukemia as a Presenting Feature of Angioimmunoblastic T-Cell Lymphoma (AITL). Front Oncol. 9: 509.
- Jain AG, Faisal-Uddin M, Khan AK, Wazir M, Shen Q, et al. (2019) Plasma cell leukemia - one in a million: A case report. World J Clin Oncol. 10(3): 161-165.
- Gundesen MT, Lund T, Moeller HEH, Abildgaard N (2019) Plasma Cell Leukemia: Definition, Presentation, and Treatment. Curr Oncol Rep. 21(1): 8.
- Gowin K, Skerget S, Keats JJ, Mikhael J, Cowan AJ (2021) Plasma cell leukemia: A review of the molecular classification, diagnosis, and evidenced-based treatment. Leuk Res. 111: 106687.
- Papadhimitriou SI, Terpos E, Liapis K, Pavlidis D, Marinakis T, et al. (2022) The Cytogenetic Profile of Primary and Secondary Plasma Cell Leukemia: Etiopathogenetic Perspectives, Prognostic Impact and Clinical Relevance to Newly Diagnosed Multiple Myeloma with Differential Circulating Clonal Plasma Cells. Biomedicines. 10(2): 209.
- Todoerti K, Agnelli L, Fabris S, Lionetti M, Tuana G, et al. (2013) Transcriptional characterization of a prospective series of primary plasma cell leukemia revealed signatures associated with tumor progression and poorer outcome. Clin Cancer Res. 19(12): 3247- 58.
- Abdallah N, Rajkumar SV, Greipp P, Kapoor P, Gertz MA, et al. (2020) Cytogenetic abnormalities in multiple myeloma: association with disease characteristics and treatment response. Blood Cancer J. 10(8): 82.
- Murase T, Inagaki A, Masaki A, Fujii K, Narita T, et al. (2019) Plasma cell myeloma positive for t(14;20) with relapse in the central nervous system. J Clin Exp Hematop. 59(3): 135-139.
- Bruserud Ø, Hansen BA, Vetti N, Johansen S, Reikvam H (2018) Successful eradication of leptomeningeal plasma cell disease. Oxf Med Case Reports. 2018(7): omy038.
- Sher T, Miller KC, Deeb G, Lee K, Chanan-Khan A (2010) Plasma cell leukaemia and other aggressive plasma cell malignancies. Br J Haematol. 150(4): 418-27.
- Tuazon SA, Holmberg LA, Nadeem O, Richardson PG (2021) A clinical perspective on plasma cell leukemia; current status and future directions. Blood Cancer J. 11(2): 23.
- Schluterman KO, Fassas AB, Van Hemert RL, Harik SI (2004) Multiple myeloma invasion of the central nervous system. Arch Neurol. 61(9): 1423–9.
- Chen CI, Masih-Khan E, Jiang H, Rabea A, Cserti-Gazdewich C, et al. (2013) Central nervous system involvement with multiple myeloma: long term survival can be achieved with radiation, intrathecal chemotherapy, and immunomodulatory agents. Br J Haematol.162(4): 483–8.
- Pagano L, Valentini CG, De Stefano V, Venditti A, Visani G, et al. (2011) Primary plasma cell leukemia: a retrospective multicenter study of 73 patients. Ann Oncol. 22(7): 1628-1635.
- Yu Z, Ogunleye F, Blankenship LM, Ward N, Ezekwudo DE, et al. (2016) Plasma Cell Leukemia Presenting with Spontaneous Tumor Lysis Syndrome: Report of a Rare Case and Literature Review. Ann Hematol Oncol. 3(12): 1125.
- Drake MB, Iacobelli S, van Biezen A, Morris C, Apperley JF, et al. (2010) European Group for Blood and Marrow Transplantation and the European Leukemia Net. Primary plasma cell leukemia and autologous stem cell transplantation. Haematologica. 95(5): 804-9.