Renu Ragupathi1, Suneesh Noushad2*
1Consultant, Oncology, Poole General Hospital, UK
2Junior Clinical Fellow, Oncology. Poole General Hospital, UK
*Corresponding Author: Suneesh Noushad, Junior Clinical Fellow, Oncology. Poole General Hospital, UK.
Abstract
Triple negative breast cancers are cancers whose cells don’t have receptors for Human Epidermal Growth Factor Receptor 2, Oestrogen and Progesterone.
Sacituzumab-Govitecan is an antibody drug conjugate used in the palliative treatment of triple-negative breast cancer. The case report is about a rare side effect of this regime.
Introduction
Triple-negative breast cancers account for 10-15% of total breast cancers [1]. Cytotoxic drugs linked to monoclonal antibodies by chemical linkers are known as antibody-drug conjugates (ADC). They deliver ultra-toxic payloads selectively to cancer cells. Sacituzumab- Govitecan is an antibody-drug conjugate (ADC) given in triple negative breast cancer patients who have had two or more chemotherapy, at least one of them for secondary breast cancer and the tumour cannot be removed by surgery. It is a combination of two drugs Sacituzumab – a monoclonal antibody and SN-38, a chemotherapy drug.
Sacitizumab acts by blocking a protein called TROP 2 on cancer cells thus reducing its dividing capacity while SN-38 destroys the tumour cells. Trop-2 is a transmembrane Calcium channel transducer that is heavily expressed in breast cancer tissue and in other tumours. The binding of the antibody component of SC to Trop-2 facilitates targeted delivery of the anti-neoplastic agent SN-38 to tumour cells. The expression of Trop 2 is associated with unfavourable prognosis in advanced breast cancer [2].
Although the remarkable efficacy of ADC has been demonstrated, they can cause adverse events in multiple organ systems such as the respiratory, digestive, circulatory, nervous and haematological systems [3]. Among these is pneumonitis which could impact ongoing treatment. Pneumonitis is defined as non-infectious causes of lung inflammation, including interstitial lung disease (ILD).
It is essential to determine the incidence of antibody-drug conjugate associated pneumonitis to prevent drug associated pneumonitis and ensure patient safety considering the seriousness of pneumonitis.
There has been a case report which reported pneumonitis caused by Trastuzumab-Deruxtecan which is an ADC [4]. There have been no case reports on Sacituzumab Govitecan associated pneumonitis.
Case Presentation
We present a case of a 60-year-old female who presented with locally advanced T4, N1, M0 breast carcinoma in January 2021. Core biopsy showed Grade 3 invasive ductal carcinoma and triple negative profile. The CT scan showed a right breast mass with involvement of the skin, suspected involvement of right pectoralis major and small lung disease load. She was treated with 6 cycles of Paclitaxel- Carboplatin. She underwent stereotactic radiosurgery for multiple cerebral and right cerebellar metastasis. She was started on first palliative line chemotherapy Epirubicin Cyclophosphamide due to disease progression.
CT scan in January 2023 showed multiple new intracranial metastasis with cerebral oedema keeping up with progression. She was started on second palliative chemotherapy Sacitizumab-Govitecan on February 2023.
Patient’s son called the hotline because the patient was increasingly getting confused 4 weeks after starting on Sacitizumab-Govitecan. She was found to be confused and not oriented to time place and person. She had associated shortness of breath and cough. SOB increased on exertion. Cough was noted to be dry. There is no history of smoking, exposure to mold, dust, asbestos, or any other chronic irritants. She had no history of asthma and COPD. She had a history of depression which was treated with paroxetine. There is no history of allergies. She had oxygen saturation of 93%. Respiratory rate was 20 breaths per minute. Blood pressure and pulse rate was found to be within normal range. Auscultation showed bilateral vesicular breath sounds. WBC count was within normal limits. Lymphocyte and monocyte count was also within normal limits. Inflammatory markers (CRP) were within normal limits. BNP was found to be normal. Blood culture and sputum culture were negative for bacterial growth.
Chest X-ray was completed to rule out chest infection.
CT Chest Abdomen and Pelvis showed new extensive ground glass consolidation within upper zones of both lungs. The changes in the lungs were suggestive of interstitial lung disease secondary to Sacituzumab-Govitecan. SG was stopped secondary to lung changes. She was started on high dose of IV methyl prednisolone (150mg) with PPI cover (proton pump inhibitor) for 3 days. Steroid was gradually tapered over 4 to 6 weeks. She was also administered IV Tazocinto cover infection, which was later switched to oral co-amoxiclav for a total of 7 days.
Shortness of breath rapidly improved with intravenous methylprednisolone. She was able to mobilise without being short of breath. CT scan done after 2 months showed complete resolution of ground glass opacity in upper zones of lungs.
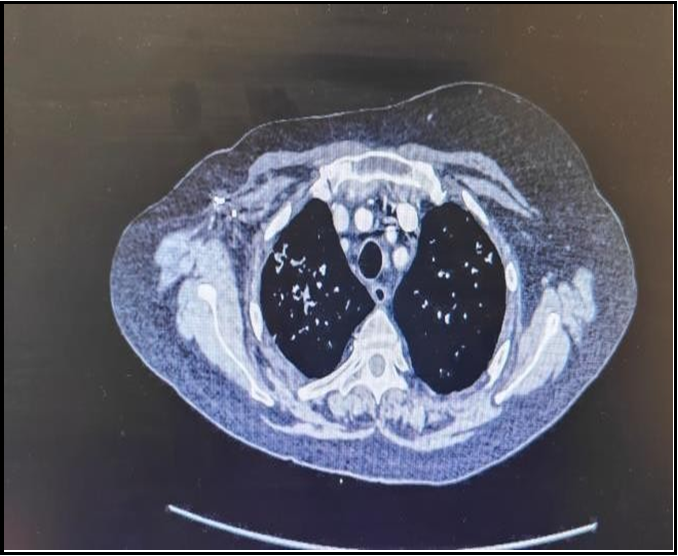
Figure A - Once the patient started developing pulmonary symptoms CT Scan of Chest was completed which showed hazy areas in the lungs. These areas have increased density. Classically it is described as ground glass opacity which indicates that the patient has developed pneumonitis secondary to the treatment with Sacituzumab Govitecan.
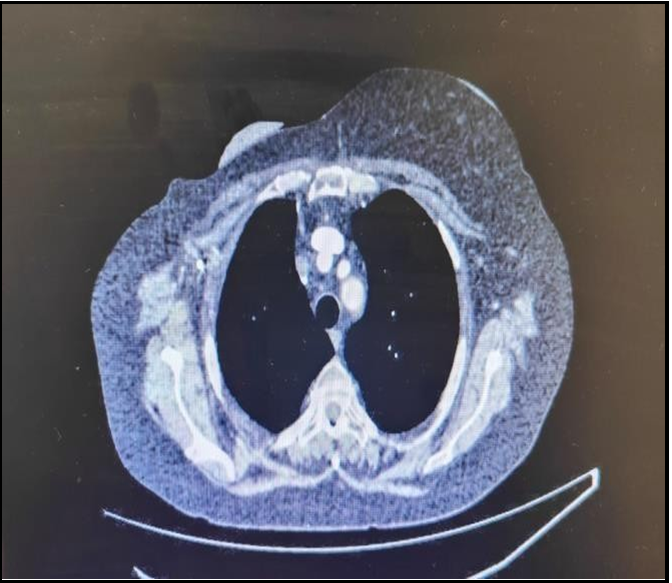
Figure B – The CT scan of lungs was completed at second month after treatment with Intravenous Steroids. It has been noted that the density is lesser appreciable compared to CT scan completed previously. During this period patient also improved symptomatically.
Discussion
In the phase 3 randomized control trial (ASCENT), [9] SG monotherapy showed improved objective response rate, progression- free survival and overall survival when compared with other single- agent chemotherapies (Eribulin, Vinorelbine, Capecitabine, or Gemcitabine) in patients with metastatic Triple Negative Breast Cancer. SG use was associated with an increased incidence of treatment-related adverse events [3] when compared to single-agent chemotherapy including grade3 or higher neutropenia, diarrhoea and febrile neutropenia. While there is an increased incidence of neutropenia and diarrhoea [5], incidence of pneumonitis is not reported.
Although the detailed mechanism of ADC induced pneumonitis has not been fully elucidated, the theoretical pathogenesis may be related to 1) low expression of ADC target antigen in normal tissues 2) the instability of linker leads to premature release of cytotoxic drugs into blood 3) cytotoxic payloads of ADCs and their drug antibody ratios 4) bystander effect of ADCs 5) drug-drug interactions.
Key risk factors that predict an increased risk of drug-induced ILD [8] include a history of pre-existing lung disease and reduced lung function, poor performance status, smoking, age older than 60 years, Japanese or African Ethnicity, and male sex. From an Oncology point of view, risk factors are multiple chemotherapy regimens, thoracic radiotherapy, the presence of lung cancer, and treatment with a combination of molecularly targeted and cytotoxic agents.
Many patients who develop ILD have no identifiable pre-existing risk factors which increased the importance of vigilance.
ADC induced ILD is a diagnosis of exclusion, and other causesof respiratory failure including cardiogenic pulmonary oedema, pneumonia and diffuse alveolar haemorrhage should be excluded.
All patients receiving SG should be monitored be monitored for signs and symptoms of ILD/Pneumonitis. If a patient develops cough, dyspnoea, fever, and a new or worsening symptom, evaluation of ILD should be started promptly. Radiological imaging should be obtained in all suspected cases, and a pulmonologist consultation should be considered.
For asymptomatic pneumonitis (grade 1), treatment should be interrupted until resolved and corticosteroids can be considered (0.5 mg/kg/day of prednisolone or equivalent). If adverse event is resolved at less than 28 days, the same dose can be maintained, and if resolved in more than 28 days, the dose is to be reduced by one level. However, if a patient develops symptomatic pneumonitis, treatment should be discontinued permanently and corticosteroid treatment should be promptly initiated (1mg/kg/day of prednisolone or equivalent) for at least 14 days followed by a gradual tapering for at least 4 weeks.
Factors that increase the risk of poor outcomes from ADC induced ILD
[8] are acute symptomatic disease with rapid symptom onset, hypoxaemia, need for mechanical ventilation, pre-existing interstitial lung disease, male sex, age over 65 years and diagnosis of small cell lung cancer.
Conclusion
The identification and monitoring of patients at risk of ADC induced ILD are crucial for timely intervention. It is important to identify the patient at risk of ADC induced ILD at an early stage by quantification of risk factors and comorbidities. When they are placed on a closer monitor after grading the risk factors treatment can be started to prevent the progression of ADC inducedILD. Unfortunately, there are no effective strategies for identification and monitoring ADC induced ILD in clinical practice beyond CT imaging and monitoring of oxygen saturation.
Statement of Ethics
This case report did not meet the threshold of research per the University Hospitals Dorset NHS Foundation Trust and thus did not require ethics approval. Verbal consent was obtained from the patient for publication of this case report and any accompanying images.
Conflicts of Interest Statement: The authors have no conflicts of interest to declare.
Funding Sources: There was no funding involved in the development of this case report.
Author Contributions: Dr Renu Ragupathi offered major contributions to the conceptualization of the case report. Interpretation, reviewing and writing completed by Dr Suneesh Noushad.
Data Availability Statement
All data generated or analysed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- Almansour NM (2022) Triple-Negative Breast Cancer: A Brief Review About Epidemiology, Risk Factors, Signaling Pathways, Treatment and Role of Artificial Intelligence. Front Mol Biosci. 9: 836417.
- Sakach E, Sacks R, Kalinsky K (2022) Trop-2 as a Therapeutic Target in Breast Cancer. Cancers (Basel). 14(23): 5936.
- Suzuki Y, Zhou S, Ota Y, Harrington M, Miyagi E, et al. (2023) Toxicity profile of antibody-drug conjugates in breast cancer: practical considerations. JNCI Cancer Spectr. 7(5): pkad069.
- Abuhelwa Z, Alloghbi A, Alqahtani A, Nagasaka M. Trastuzumab Deruxtecan-Induced Interstitial Lung Disease/Pneumonitis in ERBB2-Positive Advanced Solid Malignancies: A Systematic Review. Drugs. 82(9): 979-987.
- Prescott AE, Ravindra A, Javed A (2022) Neutropaenic Enterocolitis: A rare side effect of Sacituzumab Govitecan. Case Rep Oncol. 15(2):687-693.
- Schlam I, Tarantino P, Tolaney SM (2023) Managing adverse events of Sacituzumab Govitecan. Expert Opin Biol Ther. 23(11): 1103-1111.
- Tarantino P, Modi S, Tolaney SM, Cortés J, Hamilton EP, et al. (2021) Interstitial Lung Disease Induced by Anti-ERBB2 Antibody-Drug Conjugates: A Review. JAMA Oncol. 7(12): 1873-1881.
- Schwaiblmair M, Behr W, Haeckel T, Märkl B, Foerg W, et al. (2012) Drug Induced Interstitial Lung Disease. Open Respir Med J. 6: 63-74.
- Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, et al. (2021) Sacituzumab Govitecan in Metastatic Triple Negative Breast Cancer. N Engl J Med. 384(16): 1529-1541.