Sunil K Vivekananda1, Shivaswamy B Sadashivaiah1, Ramesh M Tambat1, Arshiya Sultana Khanum1, Nitish A Golasangi1, Mahesh Raj Joshi,2 Ranjitha Devashya3*, Jeet Bahadur Moktan4
1Department of General Surgery, Sapthagiri Institute of Medical Sciences, Bangalore, Karnataka, India.
2Clinical Pharmacist, Nepal National Hospital, Kalanki, Kathmandu, Nepal.
3Department of Pharmacy Practice, Sri Adichunchanagiri College of Pharmacy, Adichunchanagiri University, B G Nagara, Karnataka, India.
4Department of Pharmacy Practice, Margdarshan College of Pharmacy, Ilkal, Karnataka, India.
*Corresponding Author: Ranjitha Devashya, Department of Pharmacy Practice, Sri Adichunchanagiri College of Pharmacy, Adichunchanagiri University, B G Nagara, Karnataka, India.
Abstract
Facial nerve schwannoma occurring within the parotid gland is a rare tumor. Schwannomas of the parotid gland are rare neoplasms with the incidence being 0.2 % to 1.5 %. Schwannoma is a slow-growing encapsulated tumor of neuroectodermal derivation that originates from the Schwann cells of the neural sheath. Many patients present with a painless, palpable facial mass. The presence of facial nerve paralysis is variable. Schwannoma of the parotid gland is rare and may be mistaken as pleomorphic adenoma. Cytological features such as small fascicles of cells and wavy spindle-shaped nuclei are helpful to distinguish this tumor from pleomorphic adenoma. Herewith, we have presented a case of Facial nerve schwannoma in a 36-year-old male patient who presented to surgery OPD with complaints of swelling in front of the ear in the parotid region (right cheek) for 10 years.
Keywords: Parotid schwannoma; Superficial conservative parotidectomy; Facial nerve; Fine Needle Aspiration Cytology (FNAC).
Introduction
The parotid gland, located beneath the external acoustic meatus, is the largest salivary gland. When infected, the gland swells; however, painless swelling for many years can be an indication of a tumor. These can be pleomorphic adenoma, Warthin tumor, mucoepidermoid carcinoma, and other salivary gland neoplasms. However, consideration should be given to the possibility of a tumor originating from structures in the same region, such as a tumor arising from the facial nerve [1]. To differentiate between the various potential aetiologies, imaging, fine needle aspiration cytology (FNAC), core needle biopsy, and intraoperative frozen section are required.
Facial nerve schwannomas occurring in the parotid region are rare and are benign encapsulated neurogenic lesions [2]. Schwannomas, also known as neurilemmomas and neurinomas, arise from Schwann cells of nerve sheaths and typically affect the head and neck region, accounting for 25 % to 40 % of all cases [3]. Although the tumor arises from the facial nerve, only 20 % of patients experience facial nerve dysfunction, hemifacial paresis, or paralysis [4]. Parotid schwannomas are occasionally diagnosed using fine needle aspiration cytology (FNAC), and the lesions are frequently misinterpreted using FNAC [5].
In this case report, we presented a rare case of schwannoma of the right parotid gland in a middle-aged man, which was slow growing, asymptomatic, and diagnosed pre-operatively on FNAC and subsequently confirmed on histo-pathological examination post-operatively.
Case history presentation
A 36-year-old man presented to the surgery department with a complaint of painless swelling in the right cheek region for 10 years. Initially, it was approximately 1cm x 1cm for which he did not seek any medical advice, and the patient noticed a gradual increase in size over the past 7 months to attain a present size of 6cm x 4cm with no other associated symptoms, neither the patient had any history suggestive of facial nerve palsy, with in-significant past, personal and family history.
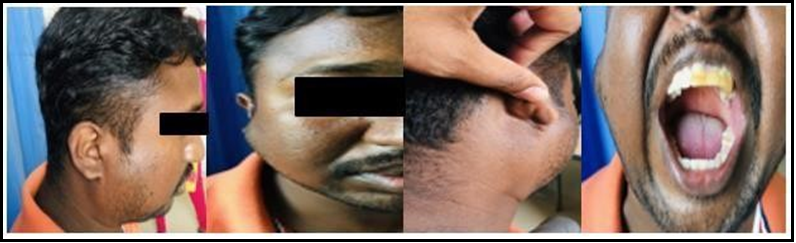
On local examination (Figure 1), a solitary, ovoid swelling of 6x4 cm, was noted in front of the right ear in the cheek, a parotid region with well-defined borders, and a smooth surface, and the skin over swollen region appears normal. Facial symmetry with the position ofthe ear lobule appears normal. No other region in the neck was swollen. On palpation, an ovoid, non-tender, cystic swelling measuring about 6x4cm with well-defined borders and a smooth surface in the right parotid region, extending vertically from 1cm below the border of the zygomatic arch to 2cm above the angle of mandible, horizontally from just anterior to tragus with curtain sign being positive. Swelling is not fixed to masseter/ adherent to overlying skin. No cervical lymphadenopathy. No features suggestive of facial nerve palsy. An oral cavity examination is normal.
The patient was subjected to specific pre-operative investigations such as FNAC which gave differentials such as benign spindle cell lesion (? Neurogenic tumor – Schwannoma /? Spindle cell lesion of salivary gland myoepithelioma/adenoma). CECT head & neck showed benign morphology exophytic nodular region right parotid gland- possibly pleomorphic adenoma. The patient was planned for superficial conservative parotidectomy.
Figure 1: Pre-operative pictures of swelling present in right parotid region
Intraoperative findings
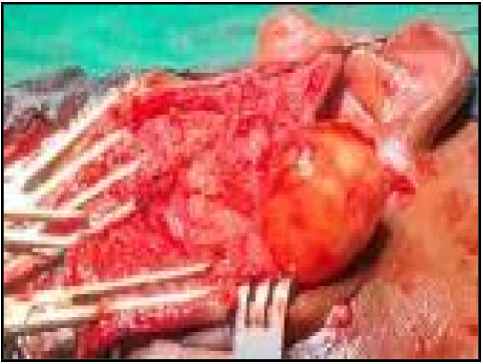
A swelling of 6x4cm was noted in the superior aspect of the superficial part of the parotid gland with variable consistency (Figure 2). Dissection was done, the trunk of the facial nerve was identified, and all the branches of the facial nerve were delineated, and tumor excised out successfully preserving all the branches of the facial nerve.
Figure 2: intraoperative pic showing tumour in superior aspect of superficial part of right parotid gland.
Histopathological examination (HPE)
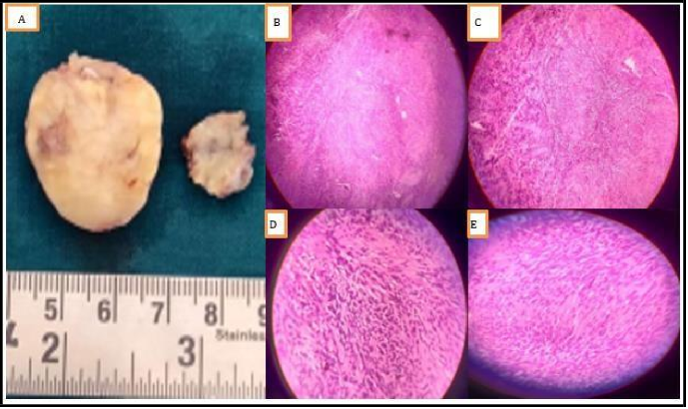
The specimen was sent for HPE which showed the following findings, grossly – specimen consists of multiple grey, soft white tissue fragments. Microscopy- encapsulated biphasic tissue shows the spindle cells arranged in nests and nodules within a loose schwannian stroma. The cells are arranged in hypocellular and cellular areas, with very bodies with thick-walled hyalinised blood vessels with cystic changes. Thus, confirming the diagnosis as Schwannoma of the right parotid.
Post-operative period
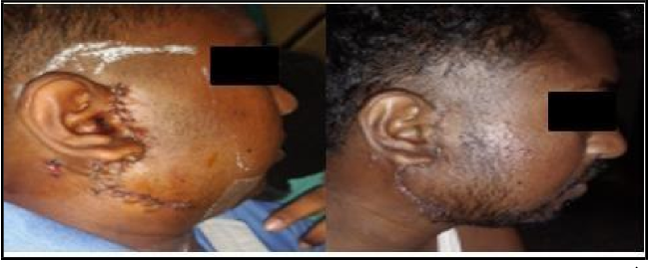
The patient was stable during discharge from the hospital. There were no signs of facial nerve palsy. The patient was followed up regularly and there were no features suggestive of facial nerve palsy (Figure 3).
Figure 3: Histopathological finding, A) Gross specimen, B) Hypocellular and hypercellular areas on 4X view, C) Verocay bodies arrangement on 10X views, D) spindle cells arrangement on 40X view, and E) Verocay bodies on 40X view.
Figure 4: Post-op period follow up pictures
Discussion
Salivary gland neoplasms account for 3% of all the neoplasms, amongst which 80 % occur in the parotid gland and the majority are identified as Pleomorphic adenoma [6]. They arise from the Schwann cells of a neural sheath of the peripheral sensory, motor, sympathetic, and cranial nerves. Schwannomas are well encapsulated, soft and whitish, yellowish or pink tumors. Sometimes they show areas of calcification and/or cystic degeneration. Their capsule is continuous with the epineurium, the most external nerve sheath [7]. Intraparotid facial nerve schwannoma was first reported by Ibarz in 1927 [8].
Approximately, amongst all reported cases of schwannomas, 25-30 % occur in the head and neck, and most of these in the eighth cranial nerve [9]. Schwannomas can be sporadic or often associated with neurofibromatosis type 2, Von Recklinghausen’s disease, and schwannomatosis and are common in post-radiation patients [10]. The most commonly affected cranial nerves are vestibular, trigeminal, and hypoglossal nerves [11]. Involvement of the facial nerve (FN) is very rare, but when it occurs, the tumor can originate at any part of the nerve, from the cerebellopontine angle to its peripheral branches. The infratemporal segment is the commonest site of development (71 %), whereas the intracarotid segment involvement is only about 9 % [3].
The prevalence of intra-parotid schwannomas ranges from 0.2 % to 1.5 % [12]. Pre-operative diagnosis of intracarotid facial nerve schwannoma is very difficult as it has low prevalence and very few typical clinical and radiological signs [2]. In a case series conducted on facial nerve schwannomas, the most common clinical presentation in intra-temporal involvement of the nerve was facial nerve dysfunction/paresis and twitching, whereas, in the extra temporal course, the most common clinical manifestation was asymptomatic parotid mass without facial paresis [13]. The peak incidence of these tumors is between the third and sixth decades even though they can occur at any age [14].
The difficulty in establishing a correct preoperative diagnosis has been pointed out by Conley and Janecka [15] because this tumor is rare and generally not suspected as preoperative facial nerve paresis is not common. FNAC is the most common investigation used preoperatively to evaluate salivary gland lesions with sensitivity and specificity, ranging from 60-100 % to 90-100 %, respectively [16].
Most parotid tumors have characteristic cytomorphologic features that help in definite preoperative diagnosis, however, a few lesions, both benign and malignant, can cause problems in interpretation [5]. Management of intracarotid schwannoma is challenging as it is linked with the most important cranial nerve. In the case of facial nerve schwannomas with infratemporal extension, a CT scan will have findings of smooth, well-circumscribed intracarotid lesion along with dilation of the fallopian canal. MRI is the gold standard investigation of choice for schwannoma which also gives detailed anatomy of facial nerves [3]. Gadolinium-enhanced MRI will demonstrate an isointense to hypointense T1 and isointense to hyperintense T2 signal with more contrast uptake [3,17]. There are no definitive radiological findings for facial nerve schwannoma [3]. There are three groups of cystic tumors in the parotid gland: non-neoplastic cysts, benign tumors with macro cystic change, and malignant tumors with macro cystic change [3,17]. The preoperative diagnosis of these cystic lesions is significant because it affects the patient’s line of management [3]. Non- neoplastic cysts are typically seen on MRI as cystic lesions with a well-defined margin and no solid component [3]. However, there are few reported cases of benign tumors with extensive cystic degeneration, which show the presence of mural nodules within the cystic component [3]. Tumor growth toward the facial canal, target sign, and string sign is the characteristic MRI features of facial nerve schwannoma that have been described in the literature [2,18]. The characteristic location of extratemporal schwannomas is posterolateral to the retromandibular vein with extension toward the stylomastoid foramen of the facial canal [13]. The target sign can be defined as the appearance of peripheral hyperintensity and central hypointensity on T2WI [3]. In schwannoma, this is due to the corresponding loose myxomatous Antoni B region in the periphery and densely cellular Antoni A region in the center seen microscopically [19]. However, the target sign is not specific as it is also seen in plexiform neurofibromas [19]. Jaiswal et al. described a string sign characterized by the presence of the parotid mass below the stylomastoid foramen with breaking into the foramen [2]. Even though pre-operatively CT, MRI, and FNAC help diagnose the tumor yet postoperative histopathological examination remains the gold standard to confirm the diagnosis.
In schwannomas, classically we see two patterns in histology; Antoni A and Antoni B. Antoni A lesions are characterized by broad interlacing ribbons of extended spindle cells with elongated nuclei arranged in waves, drifts, and whorls. On cross-section, these cylindrical cells produce a palisading pattern of nuclei about a central mass of cytoplasm called a Verocay body [20] Antoni B pattern is made up of very loose tissue, lacking the arrangement in the bundle and palisades, and is thought to be a degenerative form of Type A with a looser texture and polymorphism of cells separated by abundant myxoid, often microcystic matrix [21]. Mitoses are usually absent and malignant transformation is rare in schwannoma.
Immunostaining for S-100 is necessary to establish the neural origin of the tumor, and smooth muscle actin (SMA) to rule out a leiomyoma [20]. The differential diagnoses of intracarotid schwannoma should include pathologies that have got spindle cells in them as the main characteristic feature such as neurofibroma, fibroblastic/myofibroblastic tumors, most frequently nodular fasciitis, and fibromatosis with an infrequent myofibromatosis, fibroma, haemangiopericytoma, solitary fibrous tumor or inflammatory pseudotumor (inflammatory myofibroblastic tumor) [20]. Intranodal palisaded myofibroblastoma should also be included in differential diagnosis [22]. Like a cystic mass of the parotid, the most differential diagnoses include retention cysts, post-traumatic sialoceles, Warthin’s tumor, mucoepidermoid carcinoma, and necrotic metastases [20].
A retrospective study conducted by Caughey et al. over a period of 38 years, focused on facial nerve schwannoma of the parotid gland. Out of a total of 3722 patients with schwannomas reviewed, only 29 were related to the facial nerve. Of this small group, only eight involved the parotid segment of the facial nerve [7,23].
As schwannomas are regarded as both benign and radioresistant, complete surgical excision of the tumors by the appropriate approach is considered the treatment of choice [24,25]. Through anatomical and pathological evaluation, Marchioni et al. [4] classified the intracarotid facial nerve schwannomas into four types. This classification helps the clinician in evaluating therapeutic and prognostic factors. Type A tumors are those resectable without damaging the facial nerve. This type of tumor rarely causes preoperative facial paralysis. Type B tumors require a partial sacrifice of the facial nerve, involving a peripheral branch or distal division. Immediate reconstruction using either a nerve graft or neurorrhaphy is usually performed, and the outcome is more dependent on the branch affected than the type of reconstruction. Type C tumors require resection of the main trunk of the facial nerve, and type D tumors require the sacrifice of the main trunk and at least one of the temporospatial or cervicofacial branches. In our case, our patient had a Type A tumor. A definitive diagnosis is made only after surgical excision with histopathological examination. Patients should be counseled before surgery regarding the common complication of injuring the facial nerve. If the patient is unwilling to undergo such resection, the option of partial resection, intraoperative diagnosis to rule out malignancy, and conservative care may be taken. The main disadvantage of this method is that frozen intraoperative biopsies are not final diagnoses, permanent studies with the help of immunohistochemistry may change the diagnosis, and lead to additional surgery. On the other hand, a false positive frozen biopsy indicating malignancy may lead to unnecessary radical surgery. Therefore, we would advise performing a whole excision where nerve preservation is achievable after a thorough conversation with the patient and performing a subtotal or partial excision when nerve dissection is challenging, with radiological follow-ups every year [4].
Gross et al. suggested en bloc tumor nerve resection with interposition nerve graft for tumors that are loosely attached to small terminal midface branches, and inseparable from preoperative HB IV-VI FN function. However, in patients with good preoperative FN function (HB I-III), watchful waiting with serial imaging or examinations should be offered. Parotid Facial nerve schwannoma (PFNS) are slow-growing tumor with low malignant potential. Hence, the principle of treatment is to preserve FN function, cosmesis, and long-term tumor control [3].
There still exists controversy regarding the treatment either to apply conservative management or surgical management. It solely depends on the treating surgeon to weigh the risk of damaging the nerve against the risks of leaving schwannoma as it is.
Conclusion
Facial nerve schwannoma does not have any definitive radiological findings. It is often misdiagnosed as pleomorphic adenomas, can compromise management decisions, and increase the risk of facial nerve damage. The goal of parotid surgery is to preserve the facial nerve and obtain tumor-free margins. These surgeries are challenging owing to their complexity and hence, thorough investigation and experienced surgeons are required to perform the surgery.
References
- Bewley AF, Azhdam AM, Borrelli M (2021) Intraparotid Facial Nerve Schwannoma Mimicking Primary Parotid Neoplasm. Ear, Nose & Throat Journal. 100(6_suppl): 881S-3S.
- Jaiswal A, Mridha AR, Nath D, Bhalla AS, Thakkar A (2015) Intracarotid facial nerve schwannoma: A case report. World J Clin Cases. 3(3): 322-6.
- Sultan Abdul Kader MI, Abdullah A, Mohamad Yunus MR, Jaafar MN, Kew TY (2022) Preoperative Challenges in Managing Intraparotid Schwannoma. Cureus. 14(1): e21392.
- Seo BF, Choi HJ, Seo KJ, Jung SN (2019) Intraparotid facial nerve schwannomas. Archives of Craniofacial Surgery. 20(1): 71.
- Bhaker P, Chatterjee D, Gochhait D, Radotra BD, Dey P (2014) Schwannoma of the parotid gland: Diagnosis by fine-needle aspiration cytology. Journal of cytology/Indian Academy of Cytologists. 31(4): 196-198.
- Tidke SS, Waknis PP, Setiya S, Kale L, Jain KM (2022) Intraparotid schwannoma–Tumour in disguise: A case report and review. Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology. 34(1): 36-9.
- Damar M, Dinç AE, Şevik Eliçora S, Bişkin S, Erten G, et al. (2016) Facial Nerve Schwannoma of Parotid Gland: Difficulties in Diagnosis and Management. Case Rep Otolaryngol. 2016: 3939685.
- Kyriakos M (1987) Pathology of selected soft tissue tumors of the head and neck: Comprehensive management of head and neck tumors. Hawley, S.E., Panje, W.R., eds. Philadelphia: WB Saunders, , pp 1261–4.
- Putney FJ, Moran JJ, Thomas GK (1964) Neurogenic tumors of the head and neck. Laryngoscope. 74: 1037–59.
- Carlson ML, Deep NL, Patel NS, Lundy LB, Tombers NM, et al. (2016) Facial nerve schwannomas: review of 80 cases over 25 years at Mayo Clinic. InMayo Clinic Proceedings. 91(11): 1563- 1576.
- Mautner VF, Lindenau M, Baser ME, Hazim W, Tatagiba M, et al. (1996) The neuroimaging and clinical spectrum of neurofibromatosis 2. Neurosurgery. 38(5): 880-6.
- Chiang CW, Chang YL, Lou PJ (2001) Multicentricity of intraparotid facial nerve schwannomas. Ann Otol Rhinol Laryngol. 110: 871-874.
- Chung SY, Kim DI, Lee BH, Yoon PH, Jeon P, et al. (1998) Facial nerve schwannomas: CT and MR findings. Yonsei Med J. 39(2): 148-153.
- Stout AP. Tumors of the Peripheral Nervous System. Section II Fascicle 6. Washington DC: AFIP; 1949. Atlas of Tumor Pathology; pp. 15–6.
- Conley J, Janecka I (1973) Neurilemmoma of the facial nerve. Plastic and reconstructive surgery. 52(1): 55-59.
- Layfield LJ, Tan P, Glasgow BJ (1987) Fine-needle aspiration of salivary gland lesions. Comparison with frozen sections and histologic findings. Arch Pathol Lab Med. 111(4): 346–53.
- Takita H, Takeshita T, Shimono T, Tanaka H, Iguchi H, et al. (2017) Cystic lesions of the parotid gland: radiologic-pathologic correlation according to the latest World Health Organization 2017 Classification of Head and Neck Tumours. Japanese journal of radiology. 35(11): 629-47.
- Shimizu K, Iwai H, Ikeda K, Sakaida N, Sawada S (2005) Intraparotid facial nerve schwannoma: a report of five cases and an analysis of MR imaging results. American journal of neuroradiology. 26(6): 1328-30.
- Murphy MD, Smith WS, Smith SE, Kransdorf M, Temple H (1999) Imaging of musculoskeletal neurogenic tumors: radiologic-pathologic correlations. Radiographics. 19(5): 1253- 80.
- Serhrouchni KI, Chbani L, Hammas N, Kamal D, El Fatemi H, et al. (2014) Two rare schwannomas of head and neck. Diagnostic Pathology. 9(1): 27.
- Sethi A, Chopra S, Passey JC, Agarwal AK (2011) Intraparotid facial nerve neurofibroma: an uncommon neoplasm. Int J Morphol. 29(3): 1054–1057.
- Kandemir NO, Barut F, Ekinci T, Karagülle C, Ozdamar SO (2010) Intranodal palisaded myofibroblastoma (intranodal hemorrhagic spindle cell tumor with amianthoid fibers): a case report and literature review. Diagn Pathol. 5: 12.
- Caughey RJ, May M, Schaitkin BM (2004) Intraparotid facial nerve schwannoma: diagnosis and management. Otolaryngology—Head and Neck Surgery. 130(5): 586-92.
- Wang B, Yuan J, Chen X, Xu H, Zhou Y, et al. (2015) Extracranial non-vestibular head and neck schwannomas. Saudi Medical Journal. 36(11): 1363-1366.
- Yafit D, Horowitz G, Vital I, Locketz G, Fliss DM (2015) An algorithm for treating extracranial head and neck schwannomas. European Archives of Oto-Rhino Laryngology. 272(8): 2035-8.